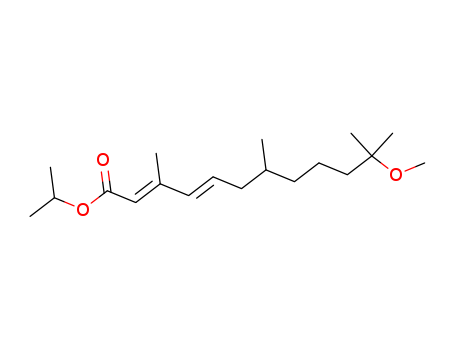
- Chemical Name:S-Methoprene
- CAS No.:40596-69-8
- Molecular Formula:C19H34O3
- Molecular Weight:310.477
- Hs Code.:2918990090
- European Community (EC) Number:613-834-0
- NSC Number:758655
- UNII:4YIQ0A94UR
- DSSTox Substance ID:DTXSID1035783
- Nikkaji Number:J344.494E
- Wikidata:Q27119791
- Metabolomics Workbench ID:56208
- ChEMBL ID:CHEMBL1875086
- Mol file:40596-69-8.mol
Synonyms:Altosid;Altosid PS 10;Altosid PS-10;Altosid PS10;Methoprene;ZR 515;ZR-515;ZR515


 Xi,
Xi,  N
N


