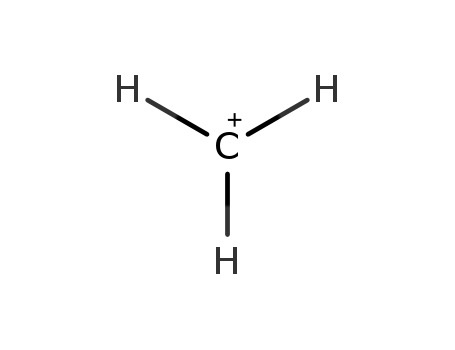
- Chemical Name:Methylium
- CAS No.:14531-53-4
- Molecular Formula:CH3
- Molecular Weight:15.0348
- Hs Code.:
- UNII:IM9JMM7N0Y
- DSSTox Substance ID:DTXSID301319120
- Nikkaji Number:J2.750.506F,J643.961F
- Wikipedia:Methenium
- Wikidata:Q3333706,Q27110071
- Mol file:14531-53-4.mol
Synonyms:methane ion (1-);methyl radical;methylium