10.1016/j.tetlet.2009.09.061
This research aimed to develop an efficient catalytic system for the aerobic epoxidation of olefins using l-oxo-bisiron(III) porphyrins [(FeIIITPP)2O] as catalysts, isobutylaldehyde as co-reductants, and dioxygen as the oxidant. The study demonstrated a significant enhancement in reactivity compared to mono-metalloporphyrin catalysts, achieving a turnover number (TON) of up to 1400 million for the catalyst. The researchers proposed a plausible mechanism involving both binuclear and mononuclear intermediates and confirmed the formation of high-valent iron intermediates through in situ UV–vis spectroscopy. The results showed that the catalytic system was highly active and selective under ambient conditions, with excellent performance across various substrates, making it a promising approach for the production of epoxides in an economical and environmentally friendly manner.
10.1007/s11426-020-9949-7
The study presents a novel method for the difunctionalization of aliphatic C(sp3)–C(sp3) bonds using dioxygen to form hydroxyketone products, which are significant in biologically active molecules, synthetic drugs, and fine chemicals. The researchers utilized a TiO2-CH3CN photocatalytic suspension system to insert dioxygen into strained cycloparaffin derivatives, resulting in the formation of β- and γ-hydroxyketone products in a one-pot reaction. Key chemicals included TiO2 (Degussa P25) as the photocatalyst, acetonitrile (CH3CN) as the solvent, and strained cycloparaffin derivatives as substrates. The purpose of these chemicals was to enable the targeted activation of inert C–H σ bonds and facilitate the insertion of both oxygen atoms from dioxygen into specific C(sp3) positions, which is a rare achievement in the field of catalytic chemistry. The study also involved isotopic labeling experiments with 18O2, Ti18O2, and H2 18O to confirm that the oxygen atoms in the hydroxyketone products originated exclusively from dioxygen, suggesting a new pathway for converting dioxygen into hydroxyketone units.



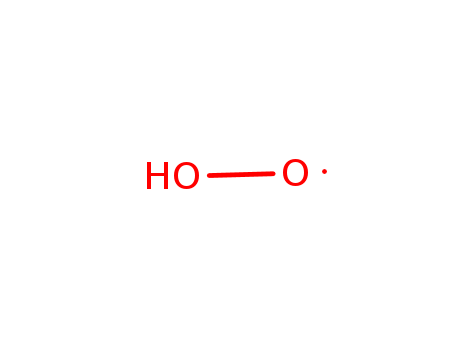
 O,
O, C
C
 O:;
O:;

