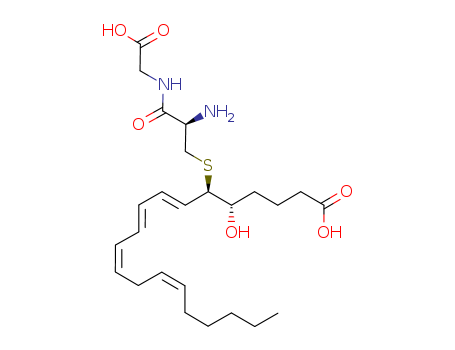
Suppliers and Price of (5S,6R,7E,9E,11Z,14Z)-6-({2-amino-2-[(carboxymethyl)carbamoyl]ethyl}sulfanyl)-5-hydroxyicosa-7,9,11,14-tetraenoic acid
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- TRC
- LTD4(LeukotrieneD4)
- 1mg
- $ 3940.00
- Sigma-Aldrich
- Leukotriene D4 ~50?μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 250 μg
- $ 1950.00
- Sigma-Aldrich
- Leukotriene D4 ~50 μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 250ug
- $ 1880.00
- Sigma-Aldrich
- Leukotriene D4 ~50?μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 10 μg
- $ 265.00
- Sigma-Aldrich
- Leukotriene D4 ~50 μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 10ug
- $ 256.00
- Sigma-Aldrich
- Leukotriene D4 ~50?μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 50 μg
- $ 796.00
- Sigma-Aldrich
- Leukotriene D4 ~50 μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 50ug
- $ 768.00
- Sigma-Aldrich
- Leukotriene D4 ~50?μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 25 μg
- $ 527.00
- Sigma-Aldrich
- Leukotriene D4 ~50 μg/mL (in methanol/ammonium acetate buffer, 70:30, pH 5.6), ≥97%
- 25ug
- $ 508.00
- Cayman Chemical
- Leukotriene D4 ≥97%
- 100μg
- $ 460.00
-
Total 15 raw suppliers


 F,
F, T
T

