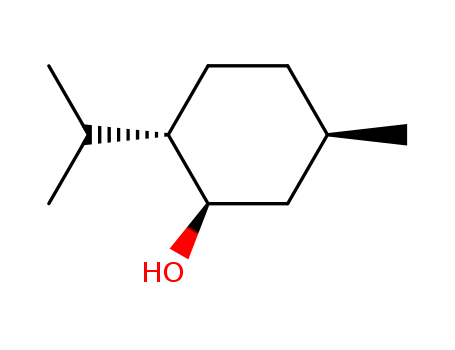
- Chemical Name:l-Menthol
- CAS No.:2216-51-5
- Deprecated CAS:15356-70-4,98167-53-4,95650-44-5
- Molecular Formula:C10H20O
- Molecular Weight:156.268
- Hs Code.:29061100
- European Community (EC) Number:201-939-0,218-690-9,624-350-4,810-475-3
- NSC Number:758395,62788,2603
- UNII:YS08XHA860,BZ1R15MTK7
- DSSTox Substance ID:DTXSID1020805,DTXSID1022180
- Nikkaji Number:J9.251G
- Wikipedia:Menthol
- Wikidata:Q407418
- NCI Thesaurus Code:C61809,C75073
- RXCUI:236388,1430390
- Pharos Ligand ID:D4NYDDUYGWT3
- Metabolomics Workbench ID:28091
- ChEMBL ID:CHEMBL470670
- Mol file:2216-51-5.mol
Synonyms:Cyclohexanol, 5-methyl-2-(1-methylethyl)-;Menthol;Menthol, (1alpha,2beta,5alpha)-Isomer


 Xi
Xi
 Xi:Irritant;
Xi:Irritant;

