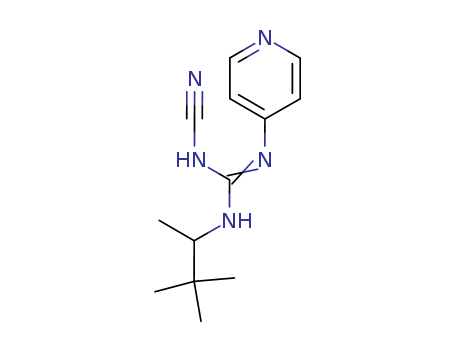
Suppliers and Price of Pinacidil monohydrate
- Business phase:
- The product has achieved commercial mass production*data from LookChem market partment
- Manufacturers and distributors:
-
- Manufacture/Brand
- Chemicals and raw materials
- Packaging
- price
- TRC
- Pinacidil monohydrate
- 500mg
- $ 445.00
- Sigma-Aldrich
- Pinacidil monohydrate powder
- 100mg
- $ 122.00
- Sigma-Aldrich
- Pinacidil monohydrate powder
- 1g
- $ 728.00
- Sigma-Aldrich
- Pinacidil monohydrate powder
- 500mg
- $ 487.00
- DC Chemicals
- Pinacidilmonohydrate(Pinacidilhydrate) >98%
- 1 g
- $ 2800.00
- CSNpharm
- PinacidilH2O
- 25mg
- $ 147.00
- ChemScene
- Pinacidil monohydrate 99.61%
- 10mg
- $ 80.00
- ChemScene
- Pinacidil monohydrate 99.61%
- 5mg
- $ 50.00
- ChemScene
- Pinacidil monohydrate 99.61%
- 25mg
- $ 160.00
- Cayman Chemical
- Pinacidil (hydrate) ≥98%
- 250mg
- $ 187.00
-
Total 39 raw suppliers


 Xn
Xn


