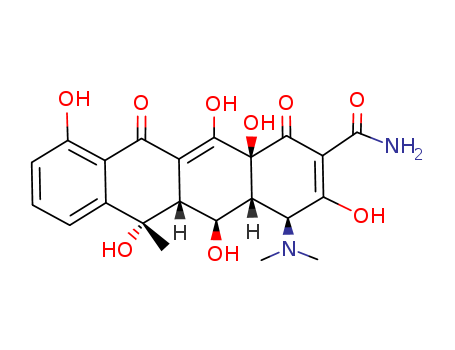
- Chemical Name:Oxytetracycline
- CAS No.:79-57-2
- Molecular Formula:C22H24N2O9
- Molecular Weight:460.441
- Hs Code.:30029090
- Mol file:79-57-2.mol
Synonyms:2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-(7CI,8CI);5-Hydroxytetracycline;Abbocin;Antibiotic TM 25;Biostat;2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-,(4S,4aR,5S,5aR,6S,12aS)-;Dabicycline;Geomycin;Geomycin (Streptomyces vimosus);Geotilin;LA 200;Lenocycline;Liquamycin LA 200;Mycoshield TMQTHC 20;NSC 9169;Oxiter 200;Oxitetracyclin;Oxycline;Oxydon;Oxylim;Oxymykoin;Oxysentin 100;Oxyterracin;Oxyterracine;Oxytetracycline amphoteric;Oxytetral;Ryomycin;Stevacin;Stevasin;Tarosin;Terrafungine;Terralon LA;Terramycin343;Terramycin Q 50;Tetran;Ursocyclin;Ursocycline;Vendarcin;


 Xn,
Xn, Xi
Xi


