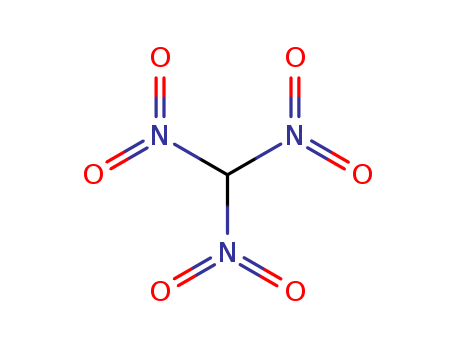
- Chemical Name:Trinitromethane
- CAS No.:517-25-9
- Deprecated CAS:57073-37-7
- Molecular Formula:CH N3 O6
- Molecular Weight:151.035
- Hs Code.:
- European Community (EC) Number:208-236-8
- UNII:Q5IR4EM1R0
- DSSTox Substance ID:DTXSID3060160
- Nikkaji Number:J2.628J
- Wikipedia:Trinitromethane
- Wikidata:Q410296
- Mol file:517-25-9.mol
Synonyms:Trinitromethane;Methane, trinitro-;NITROFORM;517-25-9;Trinitromethane [Forbidden];EINECS 208-236-8;UNII-Q5IR4EM1R0;BRN 1708361;Q5IR4EM1R0;4-01-00-00107 (Beilstein Handbook Reference);nitro form;CHN3O6;TRINITROMETHANE [MI];SCHEMBL502161;DTXSID3060160;C-H-N3-O6;AKOS006279024;LS-90436;Q410296