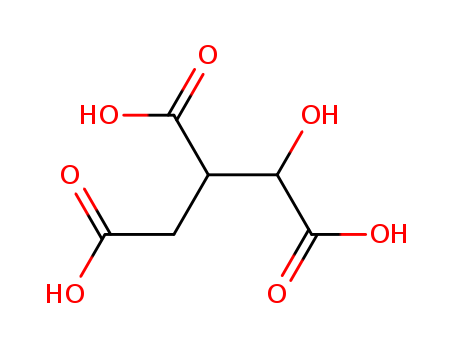
- Chemical Name:3-carboxy-2,3-dideoxy-1-hydroxypropan-1,2,3-tricarboxylic acid
- CAS No.:320-77-4
- Molecular Formula:C6H8 O7
- Molecular Weight:192.125
- Hs Code.:
- European Community (EC) Number:206-282-3
- NSC Number:203797
- UNII:9RW6G5D4MQ
- DSSTox Substance ID:DTXSID60861871
- Nikkaji Number:J95.796H
- Wikipedia:Isocitric_acid
- Wikidata:Q288927
- Metabolomics Workbench ID:142389
- ChEMBL ID:CHEMBL539669
- Mol file:320-77-4.mol
Synonyms:Isocitricacid (8CI); 1-Hydroxy-1,2,3-propanetricarboxylic acid