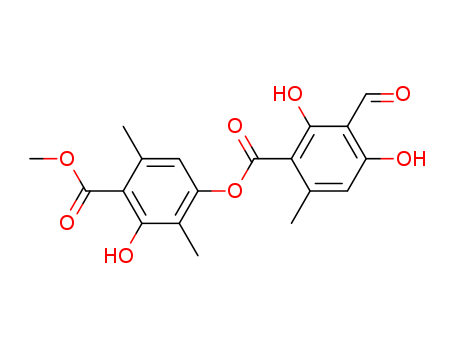
- Chemical Name:Atranorin
- CAS No.:479-20-9
- Molecular Formula:C19H18 O8
- Molecular Weight:374.347
- Hs Code.:
- European Community (EC) Number:207-527-7
- NSC Number:685591,249980,87512
- UNII:450U2VJ2VG
- DSSTox Substance ID:DTXSID10197319
- Nikkaji Number:J12.600D
- Wikipedia:Atranorin
- Wikidata:Q31749378
- Metabolomics Workbench ID:113666
- ChEMBL ID:CHEMBL173395
- Mol file:479-20-9.mol
Synonyms:atranorin;atranorin monopotassium