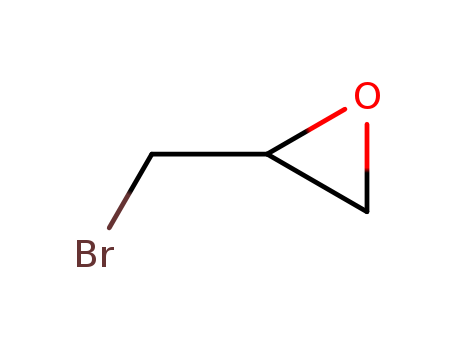
- Chemical Name:Epibromohydrin
- CAS No.:3132-64-7
- Deprecated CAS:82584-73-4
- Molecular Formula:C3H5BrO
- Molecular Weight:136.976
- Hs Code.:29109000
- European Community (EC) Number:221-525-3
- NSC Number:630
- UN Number:2558
- UNII:8E89677I6B
- DSSTox Substance ID:DTXSID5025237
- Nikkaji Number:J2.173C
- Wikidata:Q27109087
- Metabolomics Workbench ID:53649
- ChEMBL ID:CHEMBL3183066
- Mol file:3132-64-7.mol
Synonyms:Epibromohydrin;3132-64-7;1-Bromo-2,3-epoxypropane;2-(Bromomethyl)oxirane;Epibromohydrine;Epibromhydrin;(Bromomethyl)oxirane;Oxirane, (bromomethyl)-;(Bromomethyl)ethylene oxide;1,2-Epoxy-3-bromopropane;3-Bromo-1,2-epoxypropane;3-bromopropylene oxide;ALPHA-EPIBROMOHYDRIN;Epibromhydrine;Propane, 1-bromo-2,3-epoxy-;Propane, 3-bromo-1,2-epoxy-;Epibromhidrina;Epibromhydrine [French];Epibromhidrina [Spanish];NSC 630;Oxirane, 2-(bromomethyl)-;(R)-Epibromohydrine;CCRIS 2620;HSDB 2717;(RS)-3-bromo-1,2-epoxypropane;2,3-epoxypropyl bromide;EINECS 221-525-3;UN2558;BRN 0079786;AI3-03546;DTXSID5025237;UNII-8E89677I6B;CHEBI:18718;NSC-630;8E89677I6B;27815-35-6;OXIRANYLMETHYL BROMIDE;Bromohydrin;2-bromomethyloxiran;Propane,2-epoxy-;2-bromomethyloxirane;2-bromomethyl-oxirane;racemic epibromohydrine;Epibromohydrin, 98%;(+/-)-epibromohydrin;2-(bromomethyl) oxirane;2-(bromomethyl)-oxirane;EPOXY BROMOPROPANE;WLN: T3OTJ B1E;Oxirano, 2-(bromometil)-;1-bromo-2,3-epoxypropane-;SCHEMBL78600;EPIBROMOHYDRIN [HSDB];(r/s)-2-bromomethyl-oxirane;DTXCID605237;NSC630;CHEMBL3183066;(.+/-.)-(Bromomethyl)oxirane;EPIBROMOHYDRIN, (+/-)-;AMY31919;Tox21_202607;BR1167;Epibromohydrin [UN2558] [Poison];MFCD00005130;NA2558;AKOS002664702;AKOS016043519;Epibromohydrin [UN2558] [Poison];LS-1175;UN 2558;(BROMOMETHYL)OXIRANE, (+/-)-;NCGC00260155-01;BP-21061;Epibromohydrin, purum, >=97.5% (GC);CAS-3132-64-7;FT-0622147;EN300-55586;3-BROMO-1,2-EPOXYPROPANE, (+/-)-;W-106908;Q27109087


 Xn
Xn

