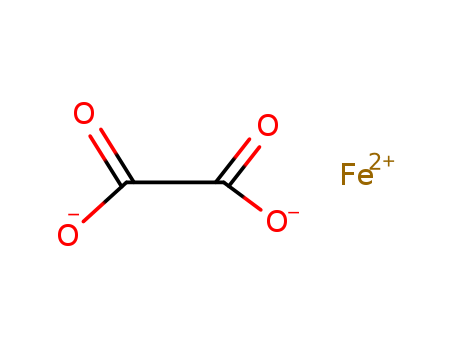
- Chemical Name:Ferrous oxalate
- CAS No.:516-03-0
- Deprecated CAS:23693-49-4,70763-81-4,736126-91-3,944409-86-3,2227313-64-4
- Molecular Formula:C2FeO4
- Molecular Weight:143.867
- Hs Code.:2917119000
- European Community (EC) Number:208-217-4
- UNII:DZP4YV3ICV
- Nikkaji Number:J415D
- Wikipedia:Iron(II) oxalate,Iron(II)_oxalate
- Wikidata:Q1990904
- Mol file:516-03-0.mol
Synonyms:FERROUS OXALATE;516-03-0;Iron(II) oxalate;Iron protoxalate;iron(2+);oxalate;Iron(2+) oxalate;FERROUSOXALATE;Iron(II) oxalate hydrate;DZP4YV3ICV;UNII-DZP4YV3ICV;EINECS 208-217-4;Iron oxalate (FeC2O4);Oxalic acid, iron(2+) salt (1:1);HSDB 463;Ethanedioic acid, iron(2+) salt (1:1);Iron, [ethanedioato(2-)-.kappa.O1,.kappa.O2]-;IRON, (ETHANEDIOATO(2-)-.KAPPA.O1,.KAPPA.O2)-;Oxalic acid iron(II);C2FeO4;C2-Fe-O4;SCHEMBL72959;FERROUS OXALATE [MI];lambda(2)-iron(2+) oxalate;FERROUS OXALATE [HSDB];FERROX (FE(C2O4));FERROUS OXALATE [MART.];FERROUS OXALATE (1:1);FERROUS OXALATE [WHO-DD];[Ethanedioato(2-)-?O1,?O2]iron;FERROUS OXALATE (FE(C2O4));OXALIC ACID, IRON(2+) SALT;AKOS015915318;IRON, (ETHANEDIOATO(2-)-O,O')-;CS-0158500;Iron, (ethanedioato(2-)-kappaO1,kappaO2)-;Q1990904