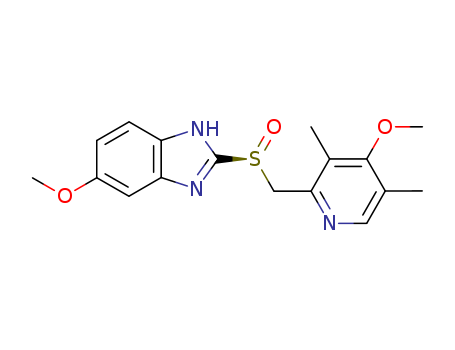
- Chemical Name:Esomeprazole
- CAS No.:119141-88-7
- Deprecated CAS:193469-77-1,326602-80-6,1116141-00-4,177541-03-6,177541-03-6,326602-80-6
- Molecular Formula:C17H19N3O3S
- Molecular Weight:345.422
- Hs Code.:29339900
- UNII:N3PA6559FT
- DSSTox Substance ID:DTXSID4044292
- Wikipedia:Esomeprazole
- Wikidata:Q553223
- NCI Thesaurus Code:C65538
- RXCUI:283742,1601995
- Pharos Ligand ID:32AV3B2YL85T
- Metabolomics Workbench ID:38698
- ChEMBL ID:CHEMBL1201320
- Mol file:119141-88-7.mol
Synonyms:Esomeprazole;Esomeprazole Magnesium;Esomeprazole Potassium;Esomeprazole Sodium;Esomeprazole Strontium;Esomeprazole Strontium Anhydrous;Nexium;Strontium, Esomeprazole