10.1039/d0ob01149e
The study presents a novel nucleophilic methylthiolation methodology that enables the incorporation of the CH3S- group into activated carbons through either conjugate additions or substitutions reactions. The researchers utilized a range of chemicals, including chalcones, acyl ester derivatives, Morita-Baylis-Hillman acetates, and methylthiomethyl esters as the primary substrates and reagents. Methanethiol, traditionally used for methylthiolation, was replaced with these novel reagents due to its flammability and toxicity. The study aimed to develop a safer, low-cost, transition-metal-free method that exhibits good group tolerance and yields moderate to excellent results. Key chemicals involved in the reaction mechanism include potassium trichloroacetate, acetic acid, and camphorsulfonic acid (CSA) as an organocatalyst. The reaction products were further utilized to synthesize sulfoxides and sulfones, demonstrating the synthetic utility of the methodology. The study also involved theoretical calculations using Density Functional Theory to investigate the reaction mechanism, confirming the role of sulfurane and sulfonium ylide as key intermediates and the importance of a Pummerer rearrangement in the formation of the reagent.


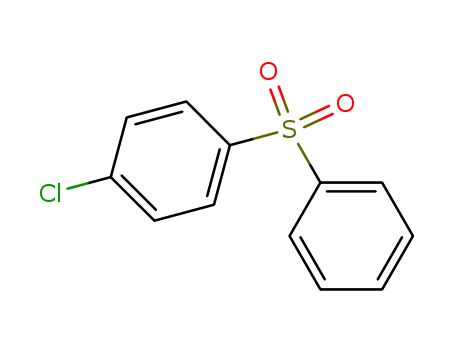
 Xn
Xn


