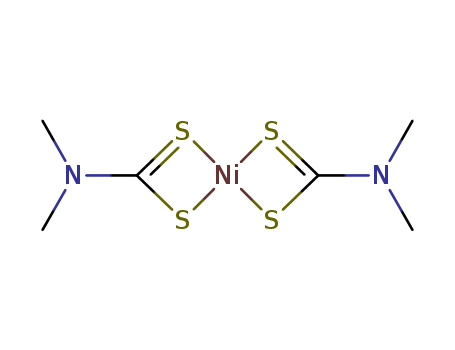
- Chemical Name:Nickel dimethyldithiocarbamate
- CAS No.:15521-65-0
- Molecular Formula:C6H12N2NiS4
- Molecular Weight:299.129
- Hs Code.:2930909090
- European Community (EC) Number:239-560-8
- Wikipedia:Nickel_bis(dimethyldithiocarbamate)
- Mol file:15521-65-0.mol
Synonyms:Nickel dimethyldithiocarbamate;Nickel bis(dimethyldithiocarbamate);N,N-dimethylcarbamodithioate;nickel(2+);N,N-Dimethyldithiocarbamate nickel;AKOS015913868