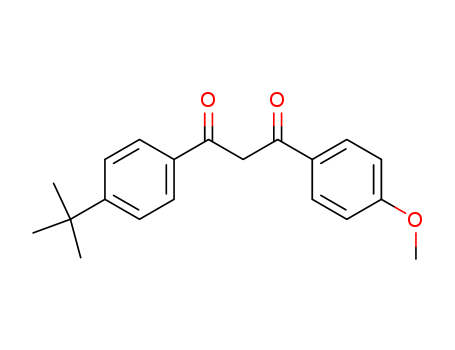
- Chemical Name:Avobenzone
- CAS No.:70356-09-1
- Deprecated CAS:87075-14-7,185160-18-3,112725-59-4,112725-59-4,185160-18-3
- Molecular Formula:C20H22O3
- Molecular Weight:310.393
- Hs Code.:29145000
- European Community (EC) Number:274-581-6
- NSC Number:758680
- UNII:G63QQF2NOX
- DSSTox Substance ID:DTXSID9044829
- Nikkaji Number:J149.841J
- Wikipedia:Avobenzone
- Wikidata:Q2775914
- NCI Thesaurus Code:C47406
- RXCUI:45045
- Pharos Ligand ID:Q93RBW2JTAV4
- Metabolomics Workbench ID:71539
- ChEMBL ID:CHEMBL1200522
- Mol file:70356-09-1.mol
Synonyms:4-tert-butyl-4'-methoxydibenzoylmethane;avobenzone;BMDBM cpd;butyl-methoxydibenzoylmethane;parsol 1789;parsol-1789


 N
N


