- Chemical Name:Arsenic
- CAS No.:7440-38-2
- Molecular Formula:AsH3
- Molecular Weight:77.9454
- Hs Code.:2804800000
- European Community (EC) Number:231-148-6
- ICSC Number:0013
- UN Number:1558,1562
- UNII:V1I29R0RJQ
- DSSTox Substance ID:DTXSID3023760,DTXSID501080836,DTXSID201080837
- Wikipedia:Arsenic
- Wikidata:Q871
- NCI Thesaurus Code:C28131
- ChEMBL ID:CHEMBL1231052
- Mol file:7440-38-2.mol
Synonyms:7440-38-2;Arsenic;As;Agent SA;Arsenic-75;Arsenowodor;Arsenicals;Arsen;Arsenwasserstoff;Gray arsenic;Grey arsenic;Arsenic Black;Metallic arsenic;Colloidal arsenic;Arsenic hydride (AsH3);Arsenic, inorganic;Arsenowodor [Polish];ARSENIC METAL;Arsenwasserstoff [German];CCRIS 55;Arsenic, elemental;HSDB 509;HSDB 510;Arsen [German,Polish];EINECS 231-148-6;EINECS 232-066-3;UN2188;UNII-V1I29R0RJQ;UN 2188;V1I29R0RJQ;UNII-N712M78A8G;CHEBI:27563;UN1558;EC 232-066-3;Arsenic 75;arsenico;arsenicum;Arsenamina;Arsenhydrid;Arsinidene;Arsina;arsenic atom;Arsin;Arsenic sponge;Arsine Gas;Inorganic arsenic;Arsine (DOT;Arsenic (Inorganic);ARSINO;ARSENIC, SOLID;ARX (CHRIS Code);ARSENIC, METALLIC;As-H3;ARSINE [MI];ARSENIC, (SOLID);ARSENICUM METALLICUM;33As;ARSENIC METAL: ARSENIA;DTXCID703886;Arsenic [UN1558] [Poison];CHEMBL1231052;DTXSID3023760;DTXSID4023886;CHEBI:35826;RQNWIZPPADIBDY-UHFFFAOYSA-N;DTXSID201080837;DTXSID501080836;N712M78A8G;Arsenic, >=99.999%, pieces;Arsine [UN2188] [Poison gas];Arsine [UN2188] [Poison gas];NA1558;NA2188;AKOS015902733;LS-1736;Arsenic, 99.999% trace metals basis;LS-21723;FT-0696579;C06269;Arsenic, powder, >=99.997% trace metals basis;ARSENIC AND INORGANIC ARSENIC COMPOUNDS (IARC);Q21060492

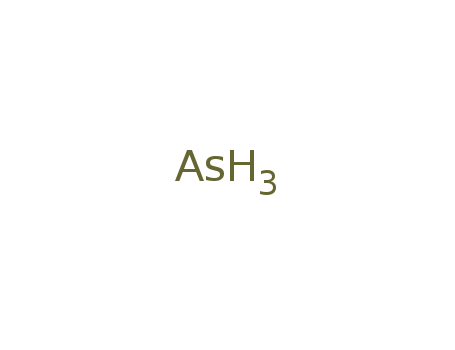

 T,
T, N
N
 T:Toxic;
T:Toxic;

