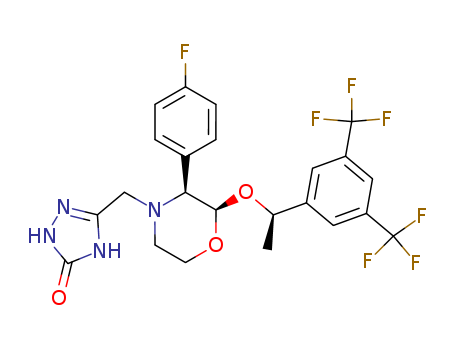
- Chemical Name:Aprepitant
- CAS No.:170729-80-3
- Molecular Formula:C23H21F7N4O3
- Molecular Weight:534.434
- Hs Code.:2934990002
- Mol file:170729-80-3.mol
Synonyms:3H-1,2,4-Triazol-3-one,5-[[2-[1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-1,2-dihydro-,[2R-[2a(R*),3a]]-;Emend;L 754030;MK 0869;MK 869;ONO7436;


 Xn,
Xn, Xi
Xi


