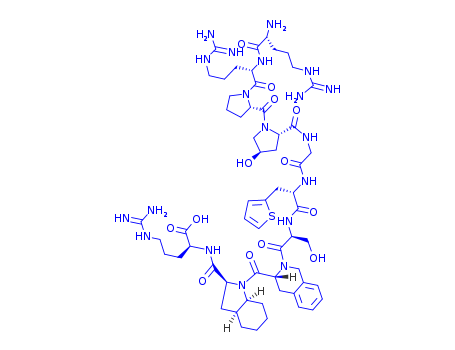
- Chemical Name:Icatibant
- CAS No.:130308-48-4
- Molecular Formula:C59H89N19O13S
- Molecular Weight:1304.54
- Hs Code.:
- European Community (EC) Number:863-000-7
- UNII:7PG89G35Q7
- DSSTox Substance ID:DTXSID20903963
- Nikkaji Number:J389.307C
- Wikipedia:Icatibant
- Wikidata:Q902379
- NCI Thesaurus Code:C98883
- RXCUI:1148138
- Pharos Ligand ID:C9RJ35J68WZH
- Metabolomics Workbench ID:149668
- ChEMBL ID:CHEMBL2028850
- Mol file:130308-48-4.mol
Synonyms:D-Arg(Hyp(3)-Thi(5)-D-Tic(7)-Oic(8))BK;D-Arg(Hyp(3)-Thi(5)-L-Tic(7)-Oic(8))BK;Firazyr;HOE 140;HOE-140;HOE140;Hoechst 140;Hoechst-140;icatibant;icatibant acetate;JE 049;JE-049;WIN 65365;WIN-65365