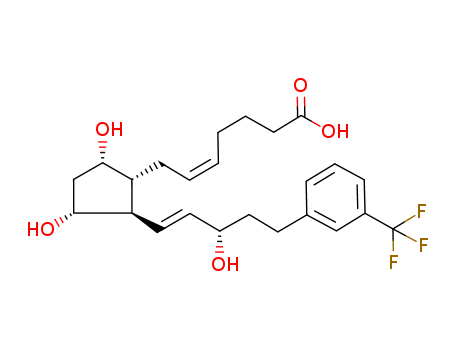
- Chemical Name:17-TRIFLUOROMETHYLPHENYL TRINOR PROSTAGLANDIN F2ALPHA
- CAS No.:221246-34-0
- Molecular Formula:C24H31F3O5
- Molecular Weight:456.502
- Hs Code.:
- Mol file:221246-34-0.mol
Synonyms:5-Heptenoicacid, 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-[3-(trifluoromethyl)phenyl]-1-pentenyl]cyclopentyl]-,(5Z)- (9CI)