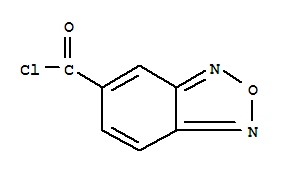
- Chemical Name:Benzofurazan-5-carbonyl chloride
- CAS No.:126147-86-2
- Molecular Formula:C7H3ClN2O2
- Molecular Weight:182.566
- Hs Code.:2934999090
- European Community (EC) Number:927-593-7,670-649-8
- DSSTox Substance ID:DTXSID60370731
- Wikidata:Q82157876
- Mol file:126147-86-2.mol
Synonyms:Benzofurazan-5-carbonyl chloride;126147-86-2;2,1,3-benzoxadiazole-5-carbonyl chloride;benzo[c][1,2,5]oxadiazole-5-carbonyl chloride;2,1,3-Benzoxadiazole-5-carbonylchloride;SCHEMBL548652;DTXSID60370731;ODCMBRSQNVPDOK-UHFFFAOYSA-N;BFA14786;MFCD00276978;AKOS006227629;PS-10756;[2,1,3]-Benzoxadiazole-5-carbonylchloride;FT-0609010;Benzo[c][1,2,5]oxadiazole-5-carbonylchloride;A805513;[2,1,3]-benzoxadiazole-5-carboxylic acid chloride;J-005339;J-519713;2-(4-AMINO-BENZENESULFONYLAMINO)-3-PHENYL-PROPIONICACID


 Xi
Xi

