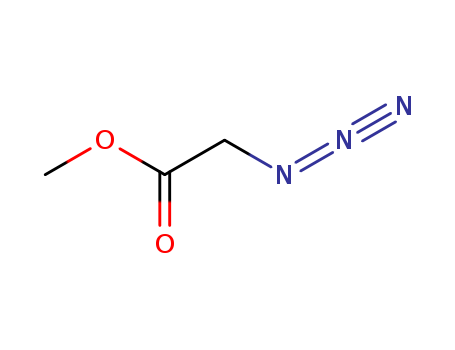
- Chemical Name:Methyl 2-azidoacetate
- CAS No.:1816-92-8
- Molecular Formula:C3H5 N3 O2
- Molecular Weight:115.092
- Hs Code.:2929909090
- European Community (EC) Number:687-878-4
- DSSTox Substance ID:DTXSID70398587
- Nikkaji Number:J1.247.582I
- Wikidata:Q82200659
- Mol file:1816-92-8.mol
Synonyms:Methyl 2-azidoacetate;1816-92-8;Methyl azidoacetate;Azidoacetic acid methyl ester;Acetic acid, 2-azido-, methyl ester;METHYLAZIDOACETATE;Acetic acid, azido-, methyl ester;azido-acetic acid methyl ester;Azidoacetic acid, methyl ester;methyl azido-acetate;methyl-2-azidoacetate;Methyl 2-azidoacetate, 97%;SCHEMBL13482799;DTXSID70398587;BCP32408;AKOS005174463;FS-4142;BB 0257904;FT-0685510;EN300-72572;V10130;J-521949;6-(2-PYRIDIN-2-YL-PYRROLIDIN-1-YL)-NICOTINICACID