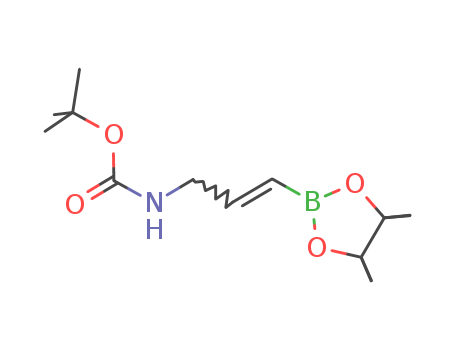
- Chemical Name:Tert-butyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)allylcarbamate
- CAS No.:468060-28-8
- Molecular Formula:C14H26BNO4
- Molecular Weight:283.176
- Hs Code.:
- DSSTox Substance ID:DTXSID60697011
- Mol file:468060-28-8.mol
Synonyms:SCHEMBL15154003;DTXSID60697011;WOLHSJKSGPKMIA-UHFFFAOYSA-N;tert-butyl 3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)allylcarbamate;SB18330;FT-0726181;tert-Butyl [3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)prop-2-en-1-yl]carbamate