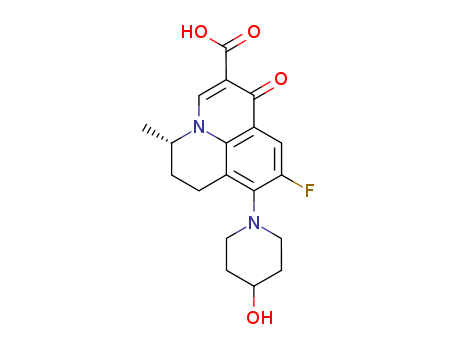
- Chemical Name:Levonadifloxacin
- CAS No.:154357-42-3
- Molecular Formula:C19H21 F N2 O4
- Molecular Weight:360.38
- Hs Code.:
- UNII:8WHH66L098
- DSSTox Substance ID:DTXSID70165599
- Nikkaji Number:J281.731D
- Wikidata:Q27117310
- NCI Thesaurus Code:C83879
- Metabolomics Workbench ID:55721
- ChEMBL ID:CHEMBL190561
- Mol file:154357-42-3.mol
Synonyms:(12S)-8-(4-((2S)-2-aminopropanoyl)oxypiperidin-1-yl)-7-fluoro-12-methyl-4-oxo-1-azatricyclo(7.3.1.05,13)trideca-2,5,7,9(13)-tetraene-3-carboxylic acid;(S)-(-)-nadifloxacin;alalevonadifloxacin;EMROK;EMROK O;L-Alanine, 1-((5S)-2-carboxy-9-fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzo(ij)quinolizin-8-yl)-4-piperidinyl ester;levonadifloxacin;WCK 2349