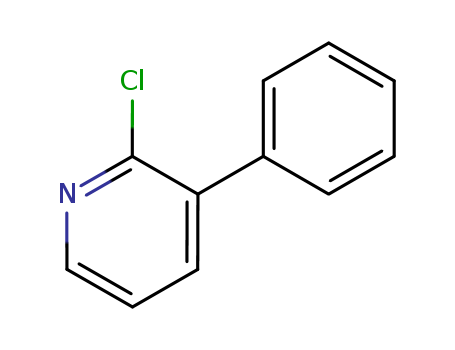
- Chemical Name:2-Chloro-3-phenylpyridine
- CAS No.:31557-57-0
- Molecular Formula:C11H8ClN
- Molecular Weight:189.644
- Hs Code.:2933399090
- DSSTox Substance ID:DTXSID90376468
- Nikkaji Number:J666.760K
- Wikidata:Q72457463
- Mol file:31557-57-0.mol
Synonyms:2-chloro-3-phenylpyridine;31557-57-0;2-chloro-3-phenyl-pyridine;SCHEMBL3327536;DTXSID90376468;GUIPMNNQJZQJFU-UHFFFAOYSA-N;MFCD04114133;AKOS006295151;CS-0194721;FT-0691612;E89738;1H-Pyrrole, 3-ethyl-2-methyl-4-(trifluoromethyl)-