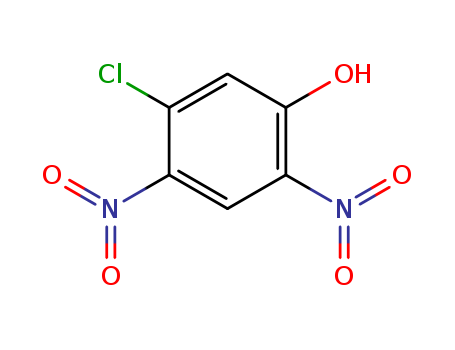
- Chemical Name:3-Chloro-4,6-dinitrophenol
- CAS No.:54715-57-0
- Molecular Formula:C6H3ClN2O5
- Molecular Weight:218.553
- Hs Code.:
- DSSTox Substance ID:DTXSID50397811
- Wikidata:Q69758000
- Mol file:54715-57-0.mol
Synonyms:5-chloro-2,4-dinitrophenol;3-Chloro-4,6-dinitrophenol;54715-57-0;5-Chlor-2,4-dinitrophenol;SCHEMBL8767757;DTXSID50397811;Phenol, 5-chloro-2,4-dinitro-;AKOS021983363