51-28-5Relevant articles and documents
Synthesis of 2,4-dinitrophenol
Khabarov, Yu.G.,Lakhmanov,Kosyakov,Ul'yanovskii
, p. 1577 - 1580 (2012)
New highly selective method is suggested for synthesis of 2,4-dinitrophenol by nitration of phenol with nitric acid in an aqueous-alcoholic medium at the boiling point of the reaction mixture. The yield of 2,4-dinitrophenol is as high as 80%. Pleiades Publishing, Ltd., 2012.
-
Carmack et al.
, p. 785,789 (1947)
-
Functionalized graphene oxide as a nanocatalyst in dephosphorylation reactions: Pursuing artificial enzymes
Orth, Elisa S.,Fonsaca, Jéssica E.S.,Almeida, Thomas Golin,Domingues, Sergio H.,Ferreira, José G.L.,Zarbin, Aldo J.G.
, p. 9891 - 9894 (2014)
The present study reports for the first time the use of a thiol-functionalized graphene oxide nanocatalyst with impressive activity (>105-fold) in dephosphorylation reactions. The innovative and recyclable nanocatalyst has potential in designing artificial enzymes with targeted multifunctionalities and in detoxification of organophosphorus agents.
Phosphodiester hydrolysis promoted by dinuclear iron(III) complexes
Piovezan, Clovis,Da Silva Lisboa, Fabio,Nunes, Fabio Souza,Drechsel, Sueli Maria
, p. 79 - 85 (2011)
We report the reactivity of three binuclear non-heme Fe(III) compounds, namely [Fe2(bbppnol)(μ-AcO)(H2O)2](ClO 4)2 (1), [Fe2(bbppnol)(μ-AcO) 2](PF6) (2), and [Fe2(bbppnol)(μ-OH)(Cl) 2]?6H2O (3), where H3bbppnol = N,N′-bis(2-hydroxybenzyl)-N,N′-bis(2-methylpyridyl)-1, 3-propanediamine-2-ol, toward the hydrolysis of bis-(2,4-dinitrophenyl)phosphate as models for phosphoesterase activity. The synthesis and characterization of the new complexes 1 and 3 was also described. The reactivity differences observed for these complexes show that the accessibility of the substrate to the reaction site is one of the key steps that determinate the hydrolysis efficiency.
Hasegawa,Abe
, p. 985,986 (1972)
Bowden,Cook
, p. 249 (1970)
An Approach to More Accurate Model Systems for Purple Acid Phosphatases (PAPs)
Bernhardt, Paul V.,Bosch, Simone,Comba, Peter,Gahan, Lawrence R.,Hanson, Graeme R.,Mereacre, Valeriu,Noble, Christopher J.,Powell, Annie K.,Schenk, Gerhard,Wadepohl, Hubert
, p. 7249 - 7263 (2015)
The active site of mammalian purple acid phosphatases (PAPs) have a dinuclear iron site in two accessible oxidation states (FeIII2 and FeIIIFeII), and the heterovalent is the active form, involved in the regulation of phosphate and phosphorylated metabolite levels in a wide range of organisms. Therefore, two sites with different coordination geometries to stabilize the heterovalent active form and, in addition, with hydrogen bond donors to enable the fixation of the substrate and release of the product, are believed to be required for catalytically competent model systems. Two ligands and their dinuclear iron complexes have been studied in detail. The solid-state structures and properties, studied by X-ray crystallography, magnetism, and M?ssbauer spectroscopy, and the solution structural and electronic properties, investigated by mass spectrometry, electronic, nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), and M?ssbauer spectroscopies and electrochemistry, are discussed in detail in order to understand the structures and relative stabilities in solution. In particular, with one of the ligands, a heterovalent FeIIIFeII species has been produced by chemical oxidation of the FeII2 precursor. The phosphatase reactivities of the complexes, in particular, also of the heterovalent complex, are reported. These studies include pH-dependent as well as substrate concentration dependent studies, leading to pH profiles, catalytic efficiencies and turnover numbers, and indicate that the heterovalent diiron complex discussed here is an accurate PAP model system.
Kinetic Study of the Aminolysis and Pyridinolysis of O-Phenyl and O-Ethyl O-(2,4-Dinitrophenyl) Thiocarbonates. A Remarkable Leaving Group Effect
Castro, Enrique A.,Cubillos, Maria,Aliaga, Margarita,Evangelisti, Sandra,Santos, Jose G.
, p. 2411 - 2416 (2004)
The reactions of a series of secondary alicyclic (SA) amines with O-phenyl and O-ethyl O-(2,4-dinitrophenyl) thiocarbonates (1 and 2, respectively) and of a series of pyridines with the former substrate are subjected to a kinetic investigation in water, at 25.0 °C, ionic strength 0.2 M (KCl). Under amine excess over the substrate, all the reactions obey pseudo-first-order kinetics and are first-order in amine. The Broensted-type plots are biphasic, with slopes (at high pKa) of β1 = 0.20 for the reactions of SA amines with 1 and 2 and β1 = 0.10 for the pyridinolysis of 1 and with slopes (at low pKa) of β2 = 0.80 for the reactions of SA amines with 1 and 2 and β2 = 1.0 for the pyridinolysis of 1. The pKa values at the curvature center (pK a0) are 7.7, 7.0, and 7.0, respectively. These results are consistent with the existence of a zwitterionic tetrahedral intermediate (T?) and a change in the rate-determining step with the variation of amine basicity. The larger pKa0 value for the pyridinolysis of 1 compared to that for 2 (pKa0 = 6.8) and the larger pKa0 value for the reactions of SA amines with 1 relative to 2 are explained by the greater inductive electron withdrawal of PhO compared to EtO. The larger pKa0 values for the reactions of SA amines with 1 and 2, relative to their corresponding pyridinolysis, are attributed to the greater nucleofugalities of SA amines compared to isobasic pyridines. The smaller pKa0 value for the reactions of SA amines with 2 than with O-ethyl S-(2,4-dinitrophenyl) dithiocarbonate (pKa0 = 9.2) is explained by the greater nucleofugality from T? of 2,4-dinitrophenoxide (DNPO-) relative to the thio derivative. The stepwise reactions of SA amines with 1 and 2, in contrast to the concerted mechanisms for the reactions of the same amines with the corresponding carbonates, is attributed to stabilization of T? by the change of O- to S-. The simple mechanism for the SA aminolysis of 2 (only one tetrahedral intermediate, T?) is in contrast to the more complex mechanism (two tetrahedral intermediates, T? and T-, the latter formed by deprotonation of T? by the amine) for the same aminolysis of the analogous thionocarbonate with 4-nitrophenoxide (NPO-) as nucleofuge. To our knowledge, this is the first example of a remarkable change in the decomposition path of a tetrahedral intermediate T? by replacement of NPO- with DNPO- as the leaving group of the substrate. This is explained by (i) the greater leaving ability from T? of DNPO- than NPO- and (ii) the similar rates of deprotonation of both T? (formed with DNPO and NPO).
Three new dinuclear nickel(II) complexes with amine pendant-armed ligands: Characterization, DFT study, antibacterial and hydrolase-like activity
Chaves, Cláudia C.V.,Farias, Giliandro,Formagio, Maíra D.,Neves, Ademir,Peralta, Rosane M.,Mikcha, Jane M.G.,de Souza, Bernardo,Peralta, Rosely A.
, (2020)
Herein, we report the synthesis and characterization of three new Ni(II) complexes, namely: [Ni2(H2LEt)(μ-OAc)2(H2O)]BPh4?ClO4 (1), (H2LEt = 2-[(N-benzyl-N-2-pyridyl methylamine)]-4-methyl-6-[N-2(pyridylmethyl)aminomethyl)]-6((2-aminoethyl)amino)methyl phenol); [Ni2(H2LProp)(μ-OAc)2(H2O)](ClO4)2 (2), (H2LProp = 2-[(N-benzyl-N-2-pyridylmethylamine)]-4-methyl-6-[N-(2-pyridylmethyl)aminomethyl]-6-((2-aminopropyl) amino)methylphenol); and [Ni2(LBut)(μ-OAc)2(H2O)](HCl)2 (3), (LBut = 2-[(N-benzyl-N-2-pyridylmethylamine)]-4-methyl-6-[N-(2-pyridylmethyl)aminomethyl]-6-((2-aminobuthyl) amino)methylphenol). All of them were characterized through spectroscopic techniques (elemental analysis, IR, UV–Vis spectroscopy), ESI-MS, electrochemistry and potentiometric titration. Density functional theory (DFT) was used to better understand the electronic and molecular structure of these complexes. The hydrolytic activity of complexes 1–3 towards the 2,4-BDNPP substrate was analyzed and complex 2 presented the highest catalytic efficiency (kcat/KM) of the three, possibly due to a greater interaction with the substrate. The complexes were also screened for their antibacterial activities using both Gram-positive and Gram-negative bacterial strains by minimum inhibitory concentration and minimum bactericidal concentration methods.
Catalysis by Cyclodextrins in Nucleophilic Aromatic Substitution Reactions
Rossi, Rita H. de,Barra, Monica,Vargas, Elba B. de
, p. 2157 - 2162 (1986)
The kinetics of the hydrolysis of 1-X-2,4-dinitrobenzene (X=Cl,F) were studied in the presence of β-cyclodextrin.The overall rate of hydrolysis is catalyzed by the added compound, and the observed catalysis is pH dependent.For the reaction of the fluoro derivative the catalytic factor at 0.01 M β-cyclodextrin changes from 7 at 10 -3 M NaOH to 2.5 at 10 -1 M NaOH.Part of the catalysis is due to nucleophilic reaction of β-cyclodextrin with the substrate and part of it is attributed to the reaction of an inclusion complex formed between the substrate and the β-cyclodextrin.The catalytic factor corresponding to the reaction in the cavity is 1.4 and 2 for the fluoro and chloro derivative, respectively
New La(III) complex immobilized on 3-aminopropyl-functionalized silica as an efficient and reusable catalyst for hydrolysis of phosphate ester bonds
Muxel, Alfredo A.,Neves, Ademir,Camargo, Maryene A.,Bortoluzzi, Adailton J.,Szpoganicz, Bruno,Castellano, Eduardo E.,Castilho, Nathalia,Bortolotto, Tiago,Terenzi, Hernan
, p. 2943 - 2952 (2014)
Described herein is the synthesis, structure, and monoesterase and diesterase activities of a new mononuclear [LaIII(L 1)(NO3)2] (1) complex (H2L 1 = 2-bis[{(2-pyridylmethyl)-aminomethyl}-6-[N-(2-pyridylmethyl) aminomethyl)])-4-methyl-6-formylphenol) in the hydrolysis of 2,4-bis(dinitrophenyl)phosphate (2,4-BDNPP). When covalently linked to 3-aminopropyl-functionalized silica, 1 undergoes disproportionation to form a dinuclear species (APS-1), whose catalytic efficiency is increased when compared to the homogeneous reaction due to second coordination sphere effects which increase the substrate to complex association constant. The anchored catalyst APS-1 can be recovered and reused for subsequent hydrolysis reactions (five times) with only a slight loss in activity. In the presence of DNA, we suggest that 1 is also converted into the dinuclear active species as observed with APS-1, and both were shown to be efficient in DNA cleavage.
MICELLAR CATALYSIS OF ORGANIC REACTIONS. PART 35. KINETIC DETERMINATION OF THE CRITICAL MICELLE CONCENTRATION OF CATIONIC MICELLES IN THE PRESENCE OF ADDITIVES
Broxton, Trevor J.,Christie, John R.,Dole, Anthony J.
, p. 437 - 441 (1994)
The critical micelle concentration of solutions of cetyltrimethylammonium bromide and of tetradecyltrimethylammonium bromides were determined by a kinetic method.This involved the determination of the rates of the hydroxydehalogenation of some activated aromatic substrates over a wide range of detergent concentrations.Measurements were made in solutions containing significant quantities of added hydroxyl ion and substrates which were themselves amphiphilic.Conventional methods cannot be applied with confidence to such systems.The effects of changing hydroxyl ion concentrations, added sodium bromide, changing the nature of the aromatic substrate (whether neutral or charged), the identity of the micellar counterion and the temperature were investigated.It was found that added bromide or hydroxyl ions resulted in a lower CMC whereas increased temperature led to an increase in the CMC.The nature of the micellar counterion (Br, F, OH, SO4) had little effect on the CMC.The presence of a charged aromatic substrate led to a considerable lowering of the CMC, whereas the neutral aromatic substrate used showed very little effect.
Bis(2,4-dinitrophenyl) phosphate hydrolysis mediated by lanthanide ions
Longhinotti, Elisane,Domingos, Josiel B.,Da Silva, Pedro L.F.,Szpoganicz, Bruno,Nome, Faruk
, p. 167 - 172 (2005)
The kinetics of the hydrolysis of bis(2,4-dinitrophenyl) phosphate (BDNPP) were studied in basic solutions in the presence of La3, Sm 3, Tb3+ and Er3. Bis-Tris propane (BTP) buffer was used to stabilize the Ln3 hydroxide complexes in solution. Two equivalents of the 2,4-dinitrophenolate ion (DNP) were liberated for each equivalent of BDNPP and the reaction showed first-order kinetics. Potentiometric titrations showed the formation of dinuclear complexes such as [Ln2(BTP)2(OH)n](6-n), with values of n varying as a function of pH, for all studied metals. Hence the catalytic effect depends on the formation of dinuclear lanthanide ion complexes with several hydroxo ligands. Copyright
DNA phosphodiester bond hydrolysis mediated by Cu(II) and Zn(II) complexes of 1,3,5,-triamino-cyclohexane derivatives
Sissi, Claudia,Mancin, Fabrizio,Palumbo, Manlio,Scrimin, Paolo,Tecilla, Paolo,Tonellato, Umberto
, p. 1265 - 1271 (2000)
The hydrolytic activity of the 1,3,5-triaminocycloxexane derivatives TACH, TACI and TMCA complexed to Zn(II) and Cu(II) towards a model phosphoric ester and plasmid DNA has been evaluated by means of spectroscopic and gel-electrophoresis techniques. At conditions close to physiological, a prominent cleavage effect mediated by the nature of the ligand and metal ion was generally observed. TACI complexes are the most active in relaxing supercoiled DNA, the effect being explained by the affinity of the hydroxylated ligand for the nucleic acid. As indicated by the dependence of cleavage efficiency upon pH, Zn(II)-complexes act by a purely hydrolytic mechanism. In the case of Cu(II)-complexes, although hydrolysis should be prominent, involvement of an oxidative pathway cannot be completely ruled out.
Effect of acetonitrile-water mixtures on the reaction of dinitrochlorobenzene and dinitrochlorobenzo-trifluoride with hydroxide ion
El-Mallah,Senior,Nabil,Ramadan,Hamed
, p. 453 - 463 (2010)
The kinetics of alkaline hydrolysis of 2-chloro-3,5-dinitrobenzotrifluoride 1 and 1-chloro-2,4-dinitrobenzene 2 were studied in various acetonitrile-water (AN-H2O) mixtures (10-90% w/w) at different temperatures. Thermodynamic parameters ΔH# and ΔS# show great variation, whereas ΔG# appears to vary little with the solvent composition presumably due to compensating variations. The results are discussed in terms of the solvent parameters such as preferential solvation, dielectric constant, polarity/polarizability, and hydrogen bond donor and acceptor parameters. It has been found that the factors controlling the reaction rates are the desolvation of OH-, the solvophobicity of the medium, and free water molecules in rich AN mixed solvent. The data showed that the solvatochromic parameters of (AN-H2O) mixed solvent are destroyed in the presence of excess OH-.
Enhancing the activation of persulfate using nitrogen-doped carbon materials in the electric field for the effective removal of: P -nitrophenol
Tang, Mengdi,Zhang, Yonggang
, p. 38003 - 38015 (2021/12/09)
Degradation of nonbiodegradable organic compounds into harmless substances is one of the main challenges in environmental protection. Electrically-activated persulfate process has served as an efficient advanced oxidation process (AOP) to degrade organic compounds. In this study, we synthesized three nitrogen-doped carbon materials, namely, nitrogen-doped activated carbon plus graphene (NC), and nitrogen-doped activated carbon (NAC), nitrogen-doped graphene (NGE), and three nitrogen-doped carbon material-graphite felt (GF) cathodes. The three nitrogen-doped carbon materials (NC, NGE, NAC) were characterized using X-ray diffraction, Raman spectroscopy, X-ray electron spectroscopy, and nitrogen desorption-adsorption. The electron spin resonance technique was used to identify the presence of hydroxyl radicals (OH), sulfate radicals (SO4-) and singlet oxygen (1O2) species. The results showed that NC was more conducive for the production of free radicals. In addition, we applied NC-GF to an electro-activated persulfate system with the degradation of p-nitrophenol and investigated its performance for contaminant degradation under different conditions. In general, the nitrogen-doped carbon electrode electro-activated persulfate process is a promising way to treat organic pollutants in wastewater.
Enhanced photocatalytic activity for 4-nitrophenol degradation using visible-light-driven In2S3/α-Fe2O3 composite
Fang, Lujuan,Huang, Xiangyang,Jiang, Ruanjing,Liu, Zijing,Munthali, Rodger Millar,Wu, Xiaogang,Zhang, Ying
, (2021/08/05)
In2S3/α-Fe2O3 composites were synthesized using the methods of hydrothermal treatment and reflux. Characterization such as X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectroscopy (XPS), and Photoluminescence (PL) was used to characterize the crystallinity and morphology of the composites, which proved the successful synthesis of the In2S3/α-Fe2O3 heterostructures. The 4-nitrophenol (PNP) degradation experiments by visible light were studied to evaluate the photocatalytic activity of the In2S3/α-Fe2O3 composites. The composites showed much higher photocatalytic degradation activities and TOC removal rate than both pure In2S3 and α-Fe2O3. The PNP degradation rate of composites was about 4.7 and 11.9 times that of pure In2S3 and α-Fe2O3, respectively. The degradation process was detected by high performance liquid chromatography and mass spectrometry, and the degradation pathway was explained. Based on the trapping experiments, e? and ?OH were the main active species and a Z-scheme photocatalytic mechanism of In2S3/α-Fe2O3 was proposed, which showed the double advantage of high redox ability and efficient electron-hole pairs separation.
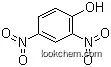
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.


