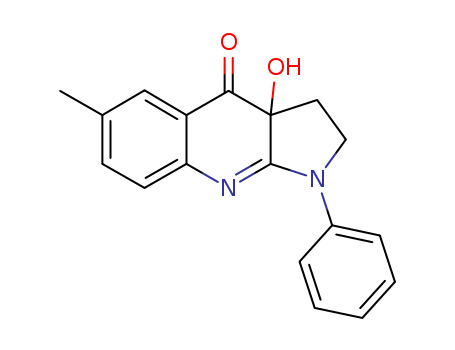
- Chemical Name:(-)-Blebbistatin
- CAS No.:856925-71-8
- Molecular Formula:C18H16N2O2
- Molecular Weight:292.337
- Hs Code.:
- European Community (EC) Number:689-630-0
- UNII:8WII7624I5
- DSSTox Substance ID:DTXSID70415329
- Nikkaji Number:J2.122.915F
- Wikidata:Q27093043
- Metabolomics Workbench ID:146559
- ChEMBL ID:CHEMBL1231358
- Mol file:856925-71-8.mol
Synonyms:(-)-Blebbistatin;856925-71-8;(S)-(-)-Blebbistatin;Blebbistatin, (-)-;(S)-blebbistatin;(-)Blebbistatin;Blebbistatin (S)-form [MI];CHEBI:75388;UNII-8WII7624I5;CHEMBL1231358;8WII7624I5;(S)-3a-hydroxy-6-methyl-1-phenyl-3,3a-dihydro-1H-pyrrolo[2,3-b]quinolin-4(2H)-one;(-)-1-PHENYL-1,2,3,4-TETRAHYDRO-4-HYDROXYPYRROLO[2,3-B]-7-METHYLQUINOLIN-4-ONE;4H-Pyrrolo(2,3-b)quinolin-4-one, 1,2,3,3a-tetrahydro-3a-hydroxy-6-methyl-1-phenyl-, (3aS)-;1,2,3,3a-Tetrahydro-3aS-hydroxy-6-methyl-1-phenyl-4H-Pyrrolo[2,3-b]quinolin-4-one;(3aS)-3a-hydroxy-6-methyl-1-phenyl-2,3-dihydropyrrolo[2,3-b]quinolin-4-one;blebbistatin-(-);(?)-Blebbistatin;MFCD08460907;blebbistatin-(+/-);SCHEMBL4330341;DTXSID70415329;EX-A703;HMS3648O21;HMS3653A05;HMS3674I13;BCP09979;(-)-Blebbistatin, solid, synthetic;BDBM50546882;s7099;AKOS024456817;CCG-267396;CS-4983;DB01944;NCGC00092288-01;NCGC00092288-02;NCGC00092288-05;AC-35899;AS-57305;BB181114;HY-13441;SW219535-1;F17375;SR-01000946255;SR-01000946255-1;Q27093043;(3aS)-3a-hydroxy-6-methyl-1-phenyl-1,2,3,3a-tetrahydro-4H-pyrrolo[2,3-b]quinolin-4-one;(3aS)-3a-hydroxy-6-methyl-1-phenyl-1H,2H,3H,3aH,4H-pyrrolo[2,3-b]quinolin-4-one;(S)-3a-Hydroxy-6-methyl-1-phenyl-1,2,3,3a-tetrahydro-4H-pyrrolo[2,3-b]quinolin-4-one;(3aS)-(-)-1,2,3,3a-Tetrahydro-3a-hydroxy-6-methyl-1-phenyl-4H-pyrrolo[2,3-b]quinolin-4-one