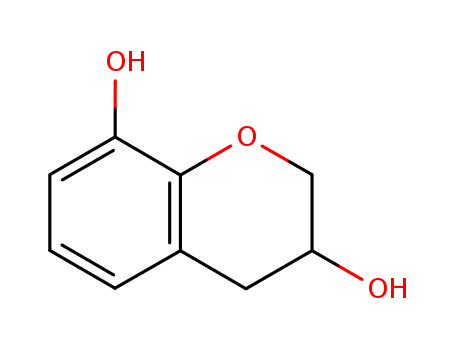
- Chemical Name:Chroman-3,8-diol
- CAS No.:81486-17-1
- Molecular Formula:C9H10O3
- Molecular Weight:166.177
- Hs Code.:2932999099
- DSSTox Substance ID:DTXSID20536634
- Nikkaji Number:J243.004E
- Mol file:81486-17-1.mol
Synonyms:Chroman-3,8-diol;81486-17-1;3,4-DIHYDRO-2H-CHROMENE-3,8-DIOL;chromane-3,8-diol;2H-1-Benzopyran-3,8-diol,3,4-dihydro-;SCHEMBL10501188;DTXSID20536634;UANJXJQTNXYKEN-UHFFFAOYSA-N;AKOS006316271;3,4-dihydro-2H-1-benzopyran-3,8-diol;3,4-Dihydro-3,8-dihydroxy-2H-1-benzopyran;G70550;A840144