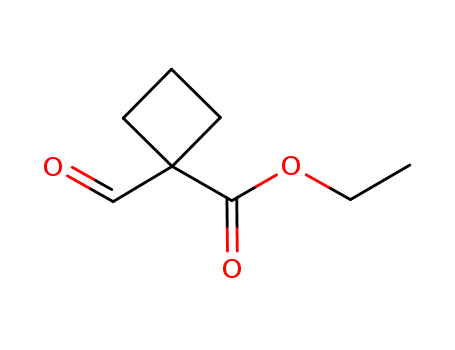
- Chemical Name:Ethyl 1-formylcyclobutanecarboxylate
- CAS No.:57742-93-5
- Molecular Formula:C8H12 O3
- Molecular Weight:156.181
- Hs Code.:2918300090
- DSSTox Substance ID:DTXSID60481311
- Wikidata:Q82316644
- Mol file:57742-93-5.mol
Synonyms:57742-93-5;ETHYL 1-FORMYLCYCLOBUTANE-1-CARBOXYLATE;ETHYL 1-FORMYLCYCLOBUTANECARBOXYLATE;Cyclobutanecarboxylic acid, 1-formyl-, ethyl ester;1-formyl-cyclobutanecarboxylic acid ethyl ester;ETHYL1-FORMYLCYCLOBUTANE-1-CARBOXYLATE;SCHEMBL1606644;DTXSID60481311;HORWHQYLTMRHBX-UHFFFAOYSA-N;AKOS015904220;CS-0380567;EN300-195038;A831577;F2147-3074;Z1255492862