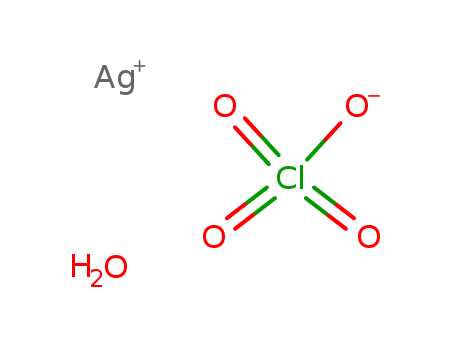
- Chemical Name:Silver perchlorate hydrate
- CAS No.:14242-05-8
- Molecular Formula:Ag. Cl H O4 . H2 O
- Molecular Weight:225.334
- Hs Code.:28432900
- European Community (EC) Number:628-990-5,677-705-0
- DSSTox Substance ID:DTXSID10931437
- Mol file:14242-05-8.mol
Synonyms:Silver perchlorate hydrate;14242-05-8;331717-44-3;silver;perchlorate;hydrate;Silverperchloratemono hydrate;MFCD00149128;Silver perchlorite;silver perchloric acid;DTXSID10931437;Silver perchlorate hydrate, 99%;Silver(I) perchlorate monohydrate;AKOS015909762;Silver(1+) perchlorate--water (1/1/1);Silver perchlorate monohydrate, 99.999% trace metals basis