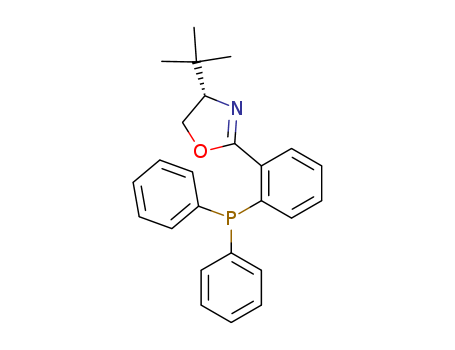
- Chemical Name:(S)-4-(tert-Butyl)-2-(2-(diphenylphosphino)phenyl)-4,5-dihydrooxazole
- CAS No.:148461-16-9
- Molecular Formula:C25H26 N O P
- Molecular Weight:387.461
- Hs Code.:2934999090
- DSSTox Substance ID:DTXSID70433043
- Nikkaji Number:J601.288D
- Wikidata:Q72470363
- Mol file:148461-16-9.mol
Synonyms:148461-16-9;(S)-4-(tert-Butyl)-2-(2-(diphenylphosphino)phenyl)-4,5-dihydrooxazole;(S)-4-TERT-BUTYL-2-[2-(DIPHENYLPHOSPHINO)PHENYL]-2-OXAZOLINE;(S)-4-tert-Butyl-2-(2-(diphenylphosphino)phenyl)-4,5-dihydrooxazole;[2-[(4S)-4-tert-butyl-4,5-dihydro-1,3-oxazol-2-yl]phenyl]-diphenylphosphane;(4S)-4-tert-Butyl-2-[2-(diphenylphosphanyl)phenyl]-4,5-dihydro-1,3-oxazole;(S)-t-BuPHOX;(S)-tBu-Phox;C25H26NOP;SCHEMBL1111225;DTXSID70433043;DMOLTNKQLUAXPI-HSZRJFAPSA-N;YFA46116;MFCD09998277;AKOS016845941;AS-72502;CS-0029503;A911337;J-008473;2-[2-(Diphenylphosphino)phenyl]-4beta-tert-butyl-2-oxazoline;(S)-4-tert-Butyl-2-[2-(diphenylphosphino)phenyl]-2-oxazoline, 97%