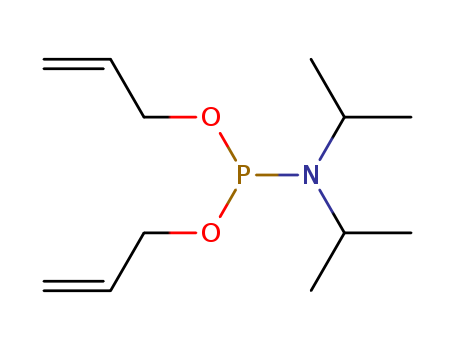
- Chemical Name:Diallyl N,N-diisopropylphosphoramidite
- CAS No.:126429-21-8
- Molecular Formula:C12H24 N O2 P
- Molecular Weight:245.302
- Hs Code.:
- European Community (EC) Number:625-730-2
- DSSTox Substance ID:DTXSID20405573
- Nikkaji Number:J1.108.929A
- Wikidata:Q82210190
- Mol file:126429-21-8.mol
Synonyms:126429-21-8;Diallyl N,N-diisopropylphosphoramidite;diallyl diisopropylphosphoramidite;N-bis(prop-2-enoxy)phosphanyl-N-propan-2-ylpropan-2-amine;Phosphoramidous acid, N,N-bis(1-methylethyl)-, di-2-propen-1-yl ester;bis(allyloxy)(diisopropylamino)phosphine;Phosphoramidous acid, bis(1-methylethyl)-, di-2-propenyl ester;SCHEMBL386395;diallyldiisopropylphosphoramidite;DTXSID20405573;QBLCHHSGJTUNSJ-UHFFFAOYSA-N;MFCD00216647;AKOS015895011;di-allyl-N,N-diisopropylphosphoramidite;Diallyl N,N-di isopropyl phosphoramidite;SY121067;DIALLYLN,N-DIISOPROPYLPHOSPHORAMIDITE;FT-0756764;E80445;A929831;J-005381