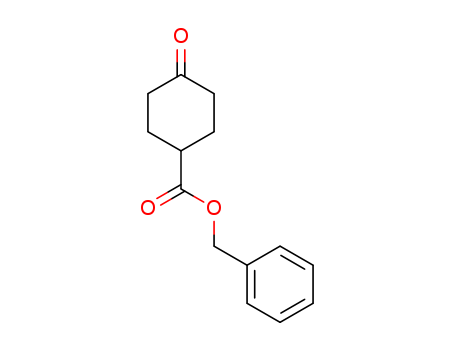
- Chemical Name:Benzyl 4-oxocyclohexanecarboxylate
- CAS No.:62596-26-3
- Molecular Formula:C14H16O3
- Molecular Weight:232.279
- Hs Code.:
- DSSTox Substance ID:DTXSID30572346
- Nikkaji Number:J994.490G
- Wikidata:Q82460654
- Mol file:62596-26-3.mol
Synonyms:benzyl 4-oxocyclohexanecarboxylate;62596-26-3;BENZYL 4-OXOCYCLOHEXANE-1-CARBOXYLATE;Cyclohexanecarboxylic acid, 4-oxo-, phenylmethyl ester;SCHEMBL850782;benzyl4-oxocyclohexanecarboxylate;DTXSID30572346;AMDOCFYEPPIDNK-UHFFFAOYSA-N;benzyl 4-oxocyclohexane carboxylate;MFCD11113219;AB10002;4-oxo-cyclohexanecarboxylic acid benzyl ester;4-ketocyclohexane carboxylic acid phenylmethyl ester;A1-07820;Cyclohexanecarboxylic acid,4-oxo-,phenylmethyl ester