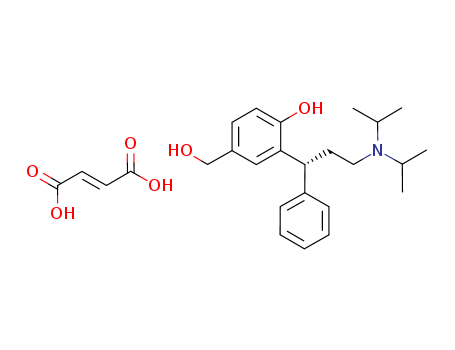
- Chemical Name:Desfesoterodine fumarate
- CAS No.:380636-50-0
- Molecular Formula:C22H31NO2.C4H4O4
- Molecular Weight:457.567
- Hs Code.:
- UNII:607O3D83YQ
- Wikidata:Q27263186
- Mol file:380636-50-0.mol
Synonyms:Desfesoterodine fumarate;PNU-200577 fumarate;5-Hydroxymethyltolterodine fumarate;UNII-607O3D83YQ;607O3D83YQ;(+)-N,N-Diisopropyl-3-(2-hydroxy-5-hydroxymethylphenyl)-3-phenylpropylamine fumarate;380636-50-0;380636-50-0 (fumarate);Benzenemethanol, 3-((1R)-3-(bis(1-methylethyl)amino)-1-phenylpropyl)-4-hydroxy-, (2E)-2-butenedioate (1:1) (salt);SCHEMBL14696972;AKOS025401643;DESFESOTERODINE FUMARATE [WHO-DD];AC-23942;Q27263186;(E)-but-2-enedioic acid;2-[(1R)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenol