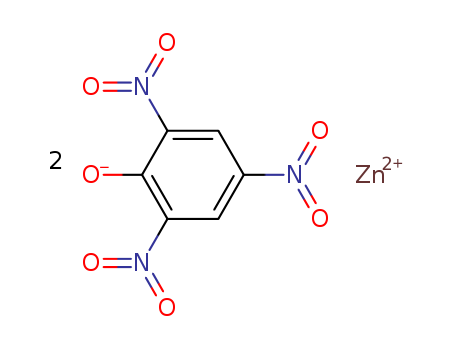
- Chemical Name:Zinc dipicrate
- CAS No.:16824-81-0
- Molecular Formula:C6H3 N3 O7 . 1/2 Zn
- Molecular Weight:521.586
- Hs Code.:
- European Community (EC) Number:240-848-0
- UNII:9JNG88GK4F
- Nikkaji Number:J236.860I
- Wikidata:Q27272635
- Mol file:16824-81-0.mol
Synonyms:Zinc dipicrate;zinc picrate;16824-81-0;zinc;2,4,6-trinitrophenolate;UNII-9JNG88GK4F;9JNG88GK4F;EINECS 240-848-0;ZINC(II) PICRATE;SCHEMBL653352;PICRIC ACID ZINC SALT (2:1);NS00085348;PHENOL, 2,4,6-TRINITRO-, ZINC SALT (2:1);Q27272635