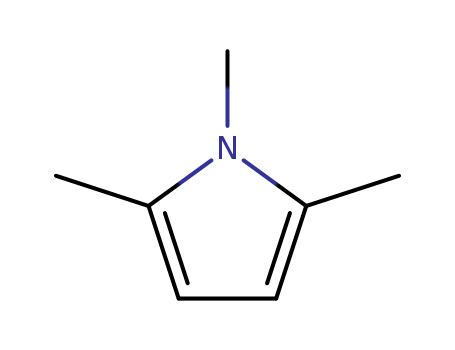
- Chemical Name:1,2,5-Trimethylpyrrole
- CAS No.:930-87-0
- Molecular Formula:C7H11N
- Molecular Weight:109.171
- Hs Code.:2933990090
- European Community (EC) Number:213-225-6
- NSC Number:81220
- UNII:7K4P5HS0RP
- DSSTox Substance ID:DTXSID20239239
- Nikkaji Number:J298.511J,J2.431.885K
- Wikidata:Q63396362
- Metabolomics Workbench ID:44012
- Mol file:930-87-0.mol
Synonyms:1,2,5-Trimethylpyrrole;930-87-0;1,2,5-Trimethyl-1H-pyrrole;1H-Pyrrole, 1,2,5-trimethyl-;7K4P5HS0RP;Pyrrole, 1,2,5-trimethyl;EINECS 213-225-6;NSC 81220;NSC-81220;MFCD00003090;NSC81220;1.2.5-Trimethylpyrrole;UNII-7K4P5HS0RP;SCHEMBL254079;1,2,5-TRIMEHYLPYRROLE;1,2,5-Trimethylpyrrole, 99%;DTXSID20239239;CHEBI:184439;1,2,5-Trimethyl-1H-pyrrole #;PYRROLE, 1,2,5-TRIMETHYL-;AKOS000101244;AS-78356;1,2,5-Trimethyl-3H-pyrrole-1-ium-3-ide;CS-0239671;FT-0606265;T1939;EN300-21742;D92550;Q63396362


 Xi
Xi


