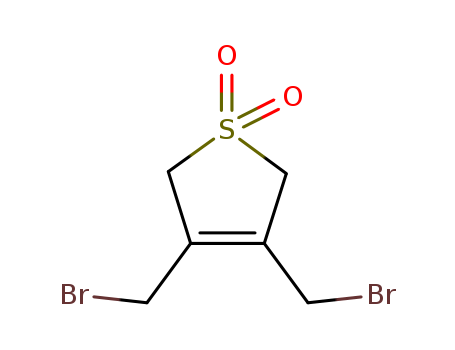
- Chemical Name:3,4-Bis(bromomethyl)-2,5-dihydrothiophene 1,1-dioxide
- CAS No.:18214-57-8
- Molecular Formula:C6H8 Br2 O2 S
- Molecular Weight:304.002
- Hs Code.:
- NSC Number:122097
- DSSTox Substance ID:DTXSID40298267
- Nikkaji Number:J3.430.098D
- Wikidata:Q82039976
- Mol file:18214-57-8.mol
Synonyms:3,4-bis(bromomethyl)-2,5-dihydrothiophene 1,1-dioxide;18214-57-8;NSC122097;starbld0009392;SCHEMBL15648455;DTXSID40298267;NSC 122097;NSC-122097;3,4-di(bromomethyl)-2,5-dihydrothiophene-1,1-dioxide