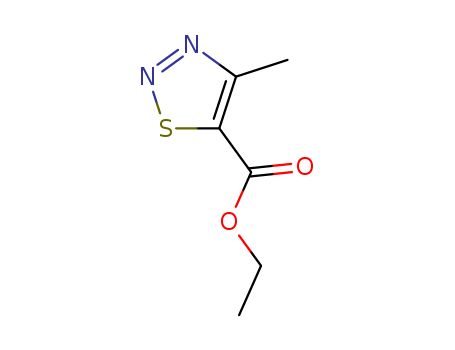
- Chemical Name:Ethyl 4-methyl-1,2,3-thiadiazole-5-carboxylate
- CAS No.:18212-20-9
- Molecular Formula:C6H8N2O2S
- Molecular Weight:172.208
- Hs Code.:2934999090
- European Community (EC) Number:624-918-1
- DSSTox Substance ID:DTXSID80374539
- Nikkaji Number:J267.176J
- Wikidata:Q72476232
- Mol file:18212-20-9.mol
Synonyms:Ethyl 4-methyl-1,2,3-thiadiazole-5-carboxylate;18212-20-9;ethyl 4-methylthiadiazole-5-carboxylate;1,2,3-Thiadiazole-5-carboxylic acid, 4-methyl-, ethyl ester;MFCD00052549;SCHEMBL2211256;4-methyl-1,2,3-thiadiazole-5-carboxylic acid ethyl ester;DTXSID80374539;STK506103;AKOS005069211;10J-523S;SY078770;CS-0045358;FT-0626095;A26562;Ethyl4-methyl-1,2,3-thiadiazole-5-carboxylate;F14572;ethyl-4-methyl-1,2,3-thiadiazole-5-carboxylate;4-methyl-5-thiadiazolecarboxylic acid ethyl ester;A812653;J-011656;J-521039;Ethyl 4-methyl-1,2,3-thiadiazole-5-carboxylate, 97%


 Xi
Xi

