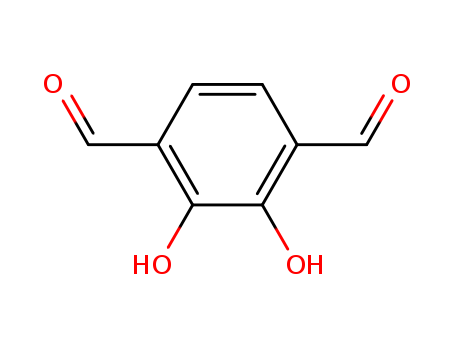
- Chemical Name:2,3-Dihydroxyterephthalaldehyde
- CAS No.:148063-59-6
- Molecular Formula:C8H6O4
- Molecular Weight:166.133
- Hs Code.:
- European Community (EC) Number:836-150-6
- Nikkaji Number:J1.612.971B
- Mol file:148063-59-6.mol
Synonyms:2,3-Dihydroxyterephthalaldehyde;148063-59-6;1,4-Benzenedicarboxaldehyde, 2,3-dihydroxy-;2,3-dihydroxybenzene-1,4-dicarbaldehyde;YSWG035;SCHEMBL17541404;YFA06359;AKOS027325677;AS-65740;CS-0110551;EN300-84424;C72817;A925073