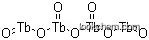12037-01-3 Usage
Description
Tetraterbium heptaoxide, also known as Terbium Oxide or Terbia, is a dark brown powder with slightly hygroscopic properties. It is insoluble in water and alkaline solutions but soluble in dilute acid. The aqueous suspension of Tetraterbium heptaoxide is alkaline and can absorb carbon dioxide from the air to form basic carbonate. It is a functional material used in the production of metal terbium, magneto-optical glass, phosphors, magneto-optical storage materials, and chemical additives.
Uses
Used in Electronics Industry:
Tetraterbium heptaoxide is used as an activator for green phosphors in color TV tubes, enhancing the display quality and color accuracy of television screens.
Used in Optics and Lasers:
Tetraterbium heptaoxide is used in special lasers and as a dopant in solid-state devices, contributing to their optical and laser properties.
Used in Fuel Cell Materials:
Tetraterbium heptaoxide is used as a dopant for crystalline solid-state devices and fuel cell materials, improving their performance and efficiency.
Used in Chemical Industry:
Tetraterbium heptaoxide acts as a precursor for the preparation of other terbium compounds, which have various applications in different industries.
Used as a Redox Catalyst:
Tetraterbium heptaoxide is used as a redox catalyst in reactions involving oxygen, facilitating chemical processes and improving reaction efficiency.
Used in Glass, Optical, and Ceramic Manufacturing:
Tetraterbium heptaoxide finds applications in the manufacture of glass, optical, and ceramic products, enhancing their properties and performance.
Used in Analytical Chemistry:
Nanoparticles of terbium oxide are used as analytical reagents for the determination of drugs in the food industry, ensuring the safety and quality of food products.
Preparation
Terbium(III,IV) oxide Synthesis: Tetraterbium nitrate or terbium carbonate or terbium sulfate solution with sodium hydroxide to form terbium hydroxide precipitate, which is filtered and burned to obtain terbium oxide.4Tb(OH)3+0.5O2→Tb4O7+6H2OTb4O7 is most often produced by ignition of the oxalate at or the sulfate in air.
Purification Methods
Dissolve it in acid (e.g. perchloric acid), precipitate it as its oxalate and ignite the oxalate at 650o.
Check Digit Verification of cas no
The CAS Registry Mumber 12037-01-3 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 1,2,0,3 and 7 respectively; the second part has 2 digits, 0 and 1 respectively.
Calculate Digit Verification of CAS Registry Number 12037-01:
(7*1)+(6*2)+(5*0)+(4*3)+(3*7)+(2*0)+(1*1)=53
53 % 10 = 3
So 12037-01-3 is a valid CAS Registry Number.
InChI:InChI=1/7O.4Tb/rO7Tb4/c1-8-5-10(3)7-11(4)6-9-2