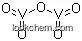
1314-62-1 Usage
Physical properties
Brown-yellow orthorhombic crystals; density 3.35 g/cm3; melts at 670°C; decomposes at 1,800°C; slightly soluble in water, 0.8g/100 mL at 20°C; soluble in concentrated acids forming an orange-yellow solution; soluble in alkalies forming vanadates.
Uses
Different sources of media describe the Uses of 1314-62-1 differently. You can refer to the following data:
1. Vanadium(V) oxide is the oxide form of vanadium. As an important vanadium compound, it is used as the major precursor to alloys of vanadium and is widely used as industrial catalyst. It can be used for the production of ferrovanadium and sulfuric acid. It can also catalyze the oxidation reaction of some anhydrides. It can also be used as a detector material in bolometer arrays for thermal imaging because of its thermal resistance. Vanadium(V) oxide is also a kind of highly selective and stable ethanol sensor materials. Moreover, the microspheres of vanadium(V) oxide formed during the synthesis of vanadium(V) oxide nanorods in a mediated polyol process exhibit excellent electrochemical properties when used as the cathode material in a lithium-ion battery.
Other applications are in making yellow glass; as a depolarizer; as a developer in photography; inhibiting UV transmission in glass;and coloring ceram; as a mordant for dyeing and printing fabrics.
2. As catalyst in the oxidation of SO2 to SO3, alcohol to acetaldehyde, etc.; for the manufacture of yellow glass; inhibiting ultraviolet light transmission in glass; depolarizer; as developer in photography; in form of ammonium vanadate as mordant in dyeing and printing fabrics and in manufacture of aniline black.
3. Vanadium pentoxide (V2O5) is a reddish-yellow powder extracted from minerals using
strong acids or alkalies. In addition to being used as a catalyst for many organic chemical
reactions, it is used in photography and in UV-protected windowpanes and to color ceramics
and dye cloth.
4. In the production of high-strength
steel alloys; catalyst in oxidation reactions; in
pesticides; in dyes and inks.
5. Vanadium(V) oxide is used in Vacuum deposition, catalysts for conversion of toluene to benzonitrile or propylene to acrylonitrile, as a detector material in bolometers and microbolometer arrays for thermal imaging.
Preparation
Vanadium pentoxide is an intermediate in recovering vanadium from minerals (See Vanadium). Sodium polyvanadate, obtained as a red cake in one of the steps in extracting vanadium from its ores is calcined at 700°C in air to form a melt of vanadium pentoxide. Pentoxide is prepared in purified form by dissolving red cake in sodium carbonate solution followed by addition of an aqueous solution of ammonia and ammonium chloride. Ammonium metavanadate is precipitated which on decomposition at 320 to 430°C forms vanadium pentoxide.
References
Liu, J, et al. "Vanadium Pentoxide Nanobelts: Highly Selective and Stable Ethanol Sensor Materials." Advanced Materials 17.17(2005): 764-767.
Cao, A. M., et al. "Self-assembled vanadium pentoxide (V2O5) hollow microspheres from nanorods and their application in lithium-ion batteries." Angewandte Chemie 44.28(2005): 4391.
Moskalyk, R. R., and A. M. Alfantazi. "Processing of vanadium: a review." Minerals Engineering 16.9 (2003): 793-805.
Khorfan, S., A. Wahoud, and Y. Reda. "Recovery of vanadium pentoxide from spent catalyst used in the manufacture of sulphuric acid." Periodica Polytechnica. Chemical Engineering 45.2 (2001): 131.
Friedrichsen, Wilhelm, and Otto Goehre. "Supported catalysts containing vanadium pentoxide and titanium dioxide and their use for the production of carboxylic acids and carboxylic anhydrides." U.S. Patent No. 3,684,741. 15 Aug. 1972.
Karunagaran, B., et al. "Study of a pulsed laser deposited vanadium oxide based microbolometer array." Smart materials and structures 12.2 (2003): 188.
Description
Vanadium pentoxide is a yellow to red colour solid and is odourless. Vanadium pentoxide
dust is the particulate form of a non-combustible, odourless, yellow-orange or dark grey
crystalline solid.
On decomposition by heating, vanadium pentoxide produces toxic fumes. Vanadium
is widely distributed in the Earth’s crust in a wide range of minerals and in fossil fuels.
Vanadium pentoxide, the major commercial product of vanadium, is mainly used in the
production of alloys with iron and aluminium. It is also used as an oxidation catalyst in
the chemical industry and in a variety of minor applications.
Chemical Properties
Different sources of media describe the Chemical Properties of 1314-62-1 differently. You can refer to the following data:
1. Vanadium pentoxide dust is an odorless, yellow
to red crystal, or powder; or fume (when vanadium is
heated). Vanadium pentoxide fume is a finely divided particulate
dispersed in air.
2. A yellow to rust-brown or orange crystals or powder. Slightly soluble in water and denser than water. Contact may cause severe irritation to skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.
Definition
vanadium pentoxide: A crystalline compound,V2O5, used extensively as a catalyst inindustrial gas-phase oxidationprocesses.
Reactivity Profile
Vanadium pentoxide is acidic in many reactions. Hence, soluble in bases. [Kirk-Othmer]. Can react with ClF3, Li, peroxyformic acid and (Ca+S+H2O). Also reacts with strong acids.
Hazard
The compound is toxic by ingestion, inhalation, and contact. Inhalation can cause asthma, cough, dyspnea, and bronchial constriction. Ingestion can cause gastrointestinal tract disturbances. Other toxic symptoms are skin pallor, greenish-black tongue, and papular skin rash (Lewis, R.J. (Sr) 1996. Sax’s Dangerous Properties of Industrial Materials, 9thed. New York: Van Nostrand Reinhold).The oral LD50 for V2O5 dust in rats is 10 mg/kg and the inhalation LCLO in rats is 70 mg/m3/2hr.
Health Hazard
Probable oral lethal dose for humans is between 5 and 50 mg/kg or between 7 drops and 1 teaspoonful for a 70 kg (150 lb.) person. Toxicity is about the same magnitude as pentavalent arsenic. A person with chronic respiratory disease is at greater risk when exposed to this substance.
Fire Hazard
Container may explode in heat of fire. When heated to decomposition, Vanadium pentoxide emits acrid smoke and fumes of vanadium oxides. Material is not flammable but Vanadium pentoxide may increase the intensity of the fire when in contact with combustible materials. Avoid chlorine trifluoride; lithium; peroxyformic acid; and calcium, sulfur, water complexes. Hazardous polymerization may not occur.
Safety Profile
Poison by ingestion,
inhalation, intraperitoneal, subcutaneous,
intratracheal, and intravenous routes. An
experimental teratogen. Human systemic
effects by inhalation: bronchiolar
constriction, including asthma, cough,
dpspnea, sputum, and conjunctiva irritation.
Experimental reproductive effects. Mutation
data reported. A respiratory irritant; causes
skin pallor, greenish-black tongue, chest
pain, cough, dyspnea, palpitation, lung
changes. When ingested it causes
gastrointestinal tract disturbances. May also
cause a papular skin rash. Mixtures with
calcium + sulfur + water may ignite
spontaneously. The absorption of V2O5 by
inhalation is nearly 100%. Incompatible with
ClF3, Li, peroxyformic acid. When heated to
decomposition it emits acrid smoke and
irritating fumes of VOx. See also
VANADIUM COMPOUNDS.
Potential Exposure
(dust); Suspected reprotoxic hazard,
Suspected of causing genetic defects, Primary irritant (w/o
allergic reaction), (fume) Possible risk of forming tumors,
Vanadium pentoxide is an industrial catalyst in oxidation
reactions; is used in glass and ceramic glazes; is a
steel additive; and is used in welding electrode coatings.
Carcinogenicity
Vanadium pentoxide was not mutagenic
in Salmonella strains and did not increase the frequency
of micronucleated erythrocytes in mice.9
In other studies vanadium compounds have produced
clear evidence of aneuploidy in somatic
cells after exposure by several different routes.
Shipping
UN2862 Vanadium pentoxide, nonfused form,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Strong acids; lithium, chlorine trifluoride;
peroxyformic acid; combustible substances.
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Vanadium pentoxide may be salvaged or disposed of in a
sanitary landfill.
Check Digit Verification of cas no
The CAS Registry Mumber 1314-62-1 includes 7 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 4 digits, 1,3,1 and 4 respectively; the second part has 2 digits, 6 and 2 respectively.
Calculate Digit Verification of CAS Registry Number 1314-62:
(6*1)+(5*3)+(4*1)+(3*4)+(2*6)+(1*2)=51
51 % 10 = 1
So 1314-62-1 is a valid CAS Registry Number.
InChI:InChI=1/5O.2V/rO5V2/c1-6(2)5-7(3)4