16789-98-3 Usage
Brand name
Concentraid (Ferring Pharmaceuticals); Ddavp
(Sanofi Aventis); Stimate (ZLB Behring).
General Description
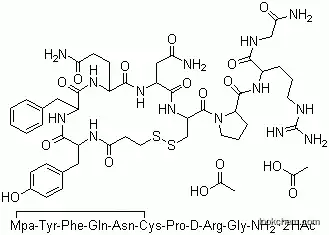
Desmopressin acetate(DDAVP, Stimate) is synthetic 1-desamino-8-D-argininevasopressin. Its efficacy, ease of administration (intranasal),long duration of action, and lack of side effects make itthe drug of choice for the treatment of central diabetesinsipidus. It may also be administered intramuscularly orintravenously. It is preferred to vasopressin injectionand oral antidiuretics for use in children. It is indicatedin the management of temporary polydipsia and polyuriaassociated with trauma to, or surgery in, the pituitary region.
Clinical Use
Desmopressin, as its acetate salt, is a synthetic analogue of vasopressin in which the N�terminal Cys is devoid of its α-amino function (1-Deamino) and where Arg8 is present as its D-isomer (D-Arg8), thus the commercial acronym DDAVP. The presence of D-Arg and
the absence of the N-terminal amine in the desmopressin structure have increased its half-life
such that it is available for oral, parenteral, or nasal use. It is used by all three of these routes
of administration to prevent or control polydipsia (excessive thirst), polyuria, and dehydration of
patients with diabetes insipidus caused by a deficiency of vasopressin. It also has been
approved for the treatment of nocturnal enuresis (bed-wetting), which is believed to be caused
by an absence of the normal night time rise in vasopressin levels.Desmopressin is known to cause an increase in both plasma factor VIII (antihemophilic factor)
and plasminogen activator. It therefore is approved by the U.S. FDA for use, parenterally and
nasally, in reducing spontaneous or trauma-induced bleeding episodes in patients with
hemophilia A and type I Von Willebrand's disease, provided that their plasma factor VIII activity is greater than 5%. Stimate, the nasal spray used in treating patients with hemophilia A and
type I Von Willebrand's disease, is 15-fold the concentration of DDAVP nasal spray; the latter is
used in treating diabetes insipidus.
Veterinary Drugs and Treatments
Desmopressin has been found to be useful in the treatment of central
diabetes insipidus
in small animals. It may be useful in treating
Von Willebrand’s disease, but its short duration of activity (2 – 4
hours) in this condition, resistance development, and expense limit
its usefulness for this disorder. Desmopressin may be useful perioperatively
to reduce lymph node involvement and metastatic disease
in canine mammary gland cancer.
Check Digit Verification of cas no
The CAS Registry Mumber 16789-98-3 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 1,6,7,8 and 9 respectively; the second part has 2 digits, 9 and 8 respectively.
Calculate Digit Verification of CAS Registry Number 16789-98:
(7*1)+(6*6)+(5*7)+(4*8)+(3*9)+(2*9)+(1*8)=163
163 % 10 = 3
So 16789-98-3 is a valid CAS Registry Number.
InChI:InChI=1/C46H64N14O12S2.2C2H4O2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25;2*1-2(3)4/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52);2*1H3,(H,3,4)
16789-98-3Relevant articles and documents
IMPROVED PROCESS FOR THE PREPARATION OF DESMOPRESSIN OR ITS PHARMACEUTICALLY ACCEPTABLE SALTS
-
, (2012/04/23)
The present invention relates to a novel and improved process for the preparation of 1-deamino-8-D-arginine vasopressin (Desmopressin) or its pharmaceutically acceptable salts thereof and also relates to an improved process for the purification of Desmopressin or its pharmaceutically acceptable salts. Further, the present invention also relates to pharmaceutical composition of Desmopressin or its pharmaceutically acceptable salts thereof.