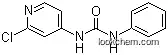68157-60-8 Usage
Description
Forchlorfenuron (68157-60-8) reversibly perturbs mammalian septin assembly, organization and function.? Has no effect on actin or tubulin polymerization.1,2 FCF induces effects on mitosis and migration which phenocopy the effects induced by septin depletion using siRNA.1 A useful tool for exploring the physiological role of septin and its complexes.3,4
Chemical Properties
Different sources of media describe the Chemical Properties of 68157-60-8 differently. You can refer to the following data:
1. White Solid
2. White to faint yellow crystalline powder.
Uses
Forchlorfenuronis is a diphenylurea-derivative cytokinin. Forchlorfenuronis is used as a plant growth regulator (PGR). Forchlorfenuronis is commonly used in horticulture to stimulate the growth of kiw
i fruit and grapes.
Agricultural Uses
Plant growth regulator: Forchlorfenuron is as a plant growth regulator to promote cell division, and to improve the quality and the
yield of fruits, especially table grapes, grape raisins, and
kiwi fruit. In some parts of California, forchlorfenuron is
said to double the size of Thompson seedless grapes and
delay crop maturity up to several weeks.
widely used in agriculture on fruits to increase their size,
Trade name
CN-11-3183; KT-30?; SKW 20010
Potential Exposure
Phenylurea/substituted urea plant
growth regulator widely used in agriculture on fruits to
increase their size, to promote cell division, and to improve
the quality and the yield of fruits, especially table grapes,
grape raisins, and kiwi fruit. In some parts of California,
forchlorfenuron is reputed to double the size of Thompson
seedless grapes and delay crop maturity up to several
weeks.
Incompatibilities
May react with strong oxidizers such as
chlorates, peroxides, nitrates, etc. Dust may form explosive
mixture with air.
Waste Disposal
Containers must be disposed
of properly by following package label directions or by
contacting your local or federal environmental control
agency, or by contacting your regional EPA office. If this
material cannot be disposed of according to label instruc tions, it may be dissolved or mixed with a combustible sol vent and burned in a chemical incinerator equipped with an
afterburner and scrubber. In accordance with 40CFR165,
follow recommendations for the disposal of pesticides and
pesticide containers.
References
1) Hu et al. (2008), Forchlorfenuron alters mammalian septin assembly, organization, and dynamics; J. Biol. Chem., 283 29563
2) DeMay et al. (2010), Cellular requirements for the small molecule forchlorfenuron to stabilize the septin cytoskeleton; Cytoskeleton, 67 383
3) Wasik et al. (2012), Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking; Mol. Biol. Cell, 23 3370
4) Vardi-Oknin et al. (2013), Forchlorfenuron disrupts SEPT9_i1 filaments and inhibits HIF-1; PLoS One, 8(8) e73179
Check Digit Verification of cas no
The CAS Registry Mumber 68157-60-8 includes 8 digits separated into 3 groups by hyphens. The first part of the number,starting from the left, has 5 digits, 6,8,1,5 and 7 respectively; the second part has 2 digits, 6 and 0 respectively.
Calculate Digit Verification of CAS Registry Number 68157-60:
(7*6)+(6*8)+(5*1)+(4*5)+(3*7)+(2*6)+(1*0)=148
148 % 10 = 8
So 68157-60-8 is a valid CAS Registry Number.
68157-60-8Relevant articles and documents
Regioselective preparation of pyridin-2-yl ureas from 2-chloropyridines catalyzed by Pd(0)
Abad, Antonio,Agullo, Consuelo,Cunat, Ana Carmen,Vilanova, Cristina
, p. 915 - 924 (2007/10/03)
The palladium-catalyzed ureidation reaction of 2-chloropyridines can be regioselectively performed in good yield, with both aryl and aliphatic ureas, using xantphos as the ligand, Pd(OAc)2 as the source of palladium, NaOt-Bu/H2O or N