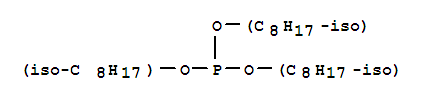Triumph International Development Limilted
- Country:
 China (Mainland)
China (Mainland) - Business License:
- Business type: Lab/Research institutions
-

- Contact Details | Similar Products


You May Like:
-
dichloroisocyanuric acid sod CAS No.: 51580-86-0
USD $100.00-150.00 / Kilogram
-
Cleves acid 1,6 CAS No.: 51548-48-2
USD $100.00-150.00 / Kilogram
-
Melamine CAS No.: 108-78-1
USD $9.00-99.00 / Kilogram
-
Faster Delivery Cesium hydro CAS No.: 35103-79-8
USD $10.00-25.00 / Kilogram
-
Musk ketone CAS 81-14-1 CAS No.: 81-14-1
USD $9.00-99.00 / Kilogram
-
-
Faster Delivery Cesium forma CAS No.: 3495-36-1
USD $10.00-25.00 / Kilogram
-
Faster Delivery Rubidium chl CAS No.: 7791-11-9
USD $10.00-25.00 / Kilogram
-
Faster Delivery CERIUM OXIDE CAS No.: 1345-13-7
USD $10.00-25.00 / Kilogram
Naphthenic Acid CAS NO.1338-24-5
- Min.Order Quantity:
- 100 Metric Ton
- Purity:
- 98%min
- Port:
- Qingdao,China
- Payment Terms:
- L/C,T/T,MoneyGram,Other
Product Details
Keywords
- 1338-24-5
- Naphthenic Acid seller in China
- Naphthenic Acid factory in China
Quick Details
- ProName: Naphthenic Acid
- CasNo: 1338-24-5
- Molecular Formula: C10H18O2
- Appearance: white powder/crystal
- Application: Used for research and industrial manuf...
- DeliveryTime: Within7-10 days after receipt of your ...
- PackAge: As customer request
- Port: Qingdao,China
- ProductionCapacity: 100kg/Month Metric Ton/Day
- Purity: 98%min
- Storage: Store in a cool,dry place and keep awa...
- Transportation: Common products:Sea/Air/Courier Dange...
- LimitNum: 100 Metric Ton
Superiority
triumph has the complete production of g- kg - mt service chain,we can make the new technology into productivity quickly in the research and development of new products.
main service
1.own made fine chemical products
2.out sourcing and quality controlling service in china
3. com for chemical synthesis
4.lab custom synthesis of api and intermadiates
quality assurance
1. nmr,hplc and coa can be supplied
2. free sample for testing
recommend products
sodium dimethyl 5-sulphonatoisophthalate
cas:1338-24-5
Details
| naphthenic acid basic information |
| product name: | naphthenic acid |
| synonyms: | naphtenic acid;naphthenic acids;naphthenic acid;agenap;carboxylicacids,naphthenic;naphid;sunapticacidb;3-naphthenic acid |
| cas: | 1338-24-5 |
| mf: | c7h10o2 |
| mw: | 126.154 |
| einecs: | 215-662-8 |
| product categories: | |
| mol file: | 1338-24-5.mol |
|
|
|
| naphthenic acid chemical properties |
| bp | 160-198 °c (6 mmhg) |
| density | 0.92 g/ml at 20 °c(lit.) |
| refractive index |
n |
| fp | 149 °c |
| freezingpoint | -30~-36℃ |
| epa substance registry system | naphthenic acids(1338-24-5) |
| safety information |
| hazard codes | xi,n |
| risk statements | 36/37/38-51/53-52-43-36/38 |
| safety statements | 26-36-61-36/37 |
| ridadr | un 3082 9/pg 3 |
| wgk germany | 2 |
| rtecs | qk8750000 |
| hazardous substances data | 1338-24-5(hazardous substances data) |
| msds information |
| provider | language |
|---|---|
| sigmaaldrich | english |
| acros | english |
| naphthenic acid usage and synthesis |
| chemical properties | clear yellowish to brown viscous liquid |
| general description | naphthenic acid is a dark colored liquid with an offensive odor. naphthenic acid is insoluble in water. naphthenic acid will burn though naphthenic acid may take some effort to ignite. the primary hazard is the threat to the environment. immediate steps should be taken to limit its spread to the environment. since naphthenic acid is a liquid naphthenic acid can easily penetrate the soil and contaminate groundwater and nearby streams. naphthenic acid is used to make paint dryers, detergents, and solvents. |
| air & water reactions | insoluble in water. |
| reactivity profile | naphthenic acid is a carboxylic acid. carboxylic acids donate hydrogen ions if a base is present to accept them. they react in this way with all bases, both organic (for example, the amines) and inorganic. their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. neutralization between an acid and a base produces water plus a salt. carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. the ph of solutions of carboxylic acids is therefore less than 7.0. many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in naphthenic acid to corrode or dissolve iron, steel, and aluminum parts and containers. carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. the reaction is slower for dry, solid carboxylic acids. insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give h2s and so3), dithionites (so2), to generate flammable and/or toxic gases and heat. their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. these reactions generate heat. a wide variety of products is possible. like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
| health hazard | principal effect is that of mild primary irritation when encountered in high concentrations. inhalation of vapor causes coughing. liquid is moderately irritating to eyes and slightly to moderately irritating to skin; excessive exposure could result in dermatitis. |
| naphthenic acid preparation products and raw materials |
| preparation products | propylene oxide-->cobalt naphthenate -->naproanilide-->emulsion oil-->j acid-->mordant black 17-->2-naphthol-6,8-disulfonic acid-->barium naphthenate-->corrosion inhibitor 581-->calcium naphthenate |
| raw materials | naphthalene-->petroleum crude oil-->naphthenic acid sodium salt-->diesel oil |
Post a RFQ