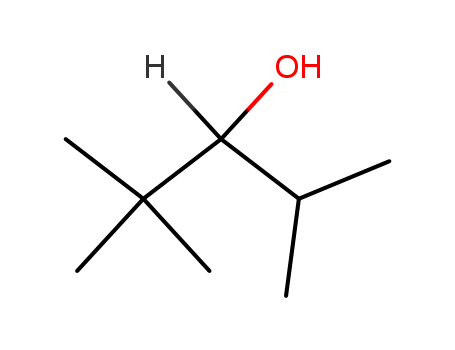
- Chemical Name:2,2,4-Trimethyl-3-pentanol
- CAS No.:5162-48-1
- Molecular Formula:C8H18O
- Molecular Weight:130.23
- Hs Code.:2905199090
- European Community (EC) Number:225-939-5
- DSSTox Substance ID:DTXSID60871117
- Nikkaji Number:J297.919E
- Wikidata:Q106956361
- Mol file:5162-48-1.mol
Synonyms:2,2,4-Trimethyl-3-pentanol;2,2,4-Trimethylpentan-3-ol;5162-48-1;3-Pentanol, 2,2,4-trimethyl-;EINECS 225-939-5;SCHEMBL2744382;DTXSID60871117;AKOS010015255;A829233;2,2,4-trimethylpentan-3-ol;1-Benzoylpiperidine-4-carboxylic acid