- 64778-78-5Benzeneethanamine,3,4-dimethoxy-a-methyl-,(aR)-
- 64779-07-3Benzenebutanoic acid, g-oxo-4-pentyl-
- 64779-10-8Benzenebutanoic acid,4-octyl-g-oxo-
- 64779-14-2Benzenebutanoic acid,4-(hexyloxy)-g-oxo-
- 64781-61-9[1,2,4]Triazino[4,3-b]indazol-4-amine,N,N-diethyl-3-phenyl-
- 647824-51-91-(tert-Butyl)-3,5-dimethyl-1H-pyrazole-4-carbaldehyde, 97%
- 647842-34-0[1,1'-Biphenyl]-4-carboxylic acid, 4'-(trifluoromethyl)-, ethyl ester
- 647843-58-12-Pyrazinamine,5-ethoxy-
- 647853-32-5Boronicacid, B-[3-(2-hydroxyethyl)phenyl]-
- 64785-96-21-Octen-3-one,1,1,2-tribromo-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
1-Butyl-3-(4-methylphenyl)sulfonylurea |
EINECS | 200-594-3 |
| CAS No. | 64-77-7 | Density | 1.184 g/cm3 |
| PSA | 83.65000 | LogP | 3.64560 |
| Solubility | 0.14g/L(25 oC) | Melting Point |
128-130°C |
| Formula | C12H18N2O3S | Boiling Point | white crystalline powder |
| Molecular Weight | 270.353 | Flash Point | N/A |
| Transport Information | N/A | Appearance | white crystalline powder |
| Safety | 26-36/37/39 | Risk Codes | 20/21/22-40-43 |
| Molecular Structure |
|
Hazard Symbols | F,Xn |
| Synonyms |
Urea,1-butyl-3-(p-tolylsulfonyl)- (8CI);1-Butyl-3-(p-methylphenylsulfonyl)urea;1-Butyl-3-(p-tolylsulfonyl)urea;3-(p-Tolyl-4-sulfonyl)-1-butylurea;Aglicid;Arkozal;Artosin;Artozin;Butamid;Butamide;D 860;Diaben;Diabetamid;Diabetol;Diabuton;Diasulfon;Dolipol;Glyconon;HLS 831;Ipoglicone;Mobenol;N-(4-Methylbenzenesulfonyl)-N'-butylurea;N-(4-Methylphenylsulfonyl)-N'-butylurea;N-(Sulfonyl-p-methylbenzene)-N'-n-butylurea;N-(p-Methylbenzenesulfonyl)-N'-butylurea;N-(p-Tolylsulfonyl)-N'-butylcarbamide;N-Butyl-N'-(4-methylphenylsulfonyl)urea;N-Butyl-N'-(p-tolylsulfonyl)urea;N-Butyl-N'-p-toluenesulfonylurea;N-n-Butyl-N'-tosylurea;NSC 23813;NSC 87833;Orabet;Oralin;Orezan;Orinase;Orinaz;Oterben;Pramidex;Rastinon;Tolbusal;Tolbutamid;Tolbutamide;Toluina;Tolumid;Tolumide;Toluvan;U 2043;Willbutamide; |
Article Data | 88 |
1-Butyl-3-(4-methylphenyl)sulfonylurea Synthetic route
- 70-55-3
toluene-4-sulfonamide

- 111-36-4
n-butyl isocyanide

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With copper(l) chloride In nitromethane at 20℃; for 2h; Catalytic behavior; Reagent/catalyst; Solvent; Milling; | 95% |
| copper(l) chloride In N,N-dimethyl-formamide for 21h; Ambient temperature; | 85% |
| With boron trifluoride diethyl etherate In diethyl ether for 2h; Ambient temperature; | 66% |
- 1792-17-2
N,N'-di-n-butylurea

- 18522-92-4
sodium p-toluenesulfonamide

A
- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

B
- 109-73-9
N-butylamine

| Conditions | Yield |
|---|---|
| at 150℃; for 8h; Product distribution; other time;; | A 94.5% B n/a |
| at 150℃; for 5h; | A 94.5% B n/a |
- 4083-64-1
Tosyl isocyanate

- 109-73-9
N-butylamine

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In neat (no solvent) at 20℃; for 0.5h; Glovebox; | 93% |
| Stage #1: Tosyl isocyanate; N-butylamine In dichloromethane at 0 - 20℃; Stage #2: With 5-hydroxymethyl-2-norbornene In dichloromethane at 0 - 20℃; Stage #3: With [{1,3-bis(mesyl)imidazolidin-2-yl}RuCl2(PCy3)(=CHPh)] In dichloromethane Heating; Further stages.; | 90% |
- 39078-72-3
S-methyl N-butylthiocarbamate

- 70-55-3
toluene-4-sulfonamide

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 110℃; for 4h; Substitution; | 85% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 110℃; | 85% |
- 3898-47-3
phenyl-N-butylcarbamate

- 70-55-3
toluene-4-sulfonamide

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile Reflux; Green chemistry; | 80% |
- 70-55-3
toluene-4-sulfonamide

- 39764-19-7
1-(1-butylaminocarbonyl)-1H-benzotriazole

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| Stage #1: toluene-4-sulfonamide; 1-(1-butylaminocarbonyl)-1H-benzotriazole at 100℃; Inert atmosphere; neat (no solvent); Stage #2: With potassium carbonate for 0.25h; Inert atmosphere; neat (no solvent); Stage #3: With hydrogenchloride In water at 60℃; for 0.5h; | 75% |
- 70-55-3
toluene-4-sulfonamide

- 27095-02-9
hydroxyethyl carbamic acid butyl ester

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide | 18% |
- 41891-17-2
N-butylcarbamoyl chloride

- 18522-92-4
sodium p-toluenesulfonamide

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide
- 4932-55-2
N-(4-methylphenylsulfonyl)-N'-butylthiourea

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide |
- 98275-71-9
N-butyl-O-methyl-isourea

- 98-59-9
p-toluenesulfonyl chloride

- 64-77-7
N-[(butylamino)carbonyl]-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| und Erwaermen des Reaktionsprodukts mit wss.HCl; |
1-Butyl-3-(4-methylphenyl)sulfonylurea Chemical Properties
IUPAC Name: 1-Butyl-3-(4-methylphenyl)sulfonylurea
CAS: 64-77-7
Formula : C12H18N2O3S
Molecular Weight: 270.35
Molecular Structure of 1-Butyl-3-(4-methylphenyl)sulfonylurea (64-77-7):
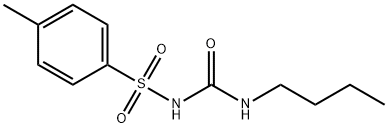
Density: 1.184 g/cm3
Melting Point: 128-130°C
Appearance: white crystalline powder
Index of Refraction: 1.532
Molar Refractivity: 70.76 cm3
Molar Volume: 228.2 cm3
Polarizability: 28.05×10-24cm3
Surface Tension: 42.7 dyne/cm
Water Solubility: 182.6(mg/L) at 25°C
Product Categories: potassium channel
1-Butyl-3-(4-methylphenyl)sulfonylurea Toxicity Data With Reference
| 1. | sce-mus-orl 28,600 µg/kg | MUREAV Mutation Research. 77 (1980),349. | ||
| 2. | sce-ham-ipr 28,600 µg/kg | MUREAV Mutation Research. 77 (1980),349. | ||
| 3. | orl-wmn LDLo:1 g/kg:GIT:SYS | ATXKA8 Archiv fuer Toxikologie. 23 (1968),153. | ||
| 4. | orl-rat LD50:2490 mg/kg | PMDCAY Progress in Medical Chemistry. 1 (1961),187. | ||
| 5. | ipr-rat LD50:860 mg/kg | FRPSAX Farmaco, Edizione Scientifica. 12 (1957),268. | ||
| 6. | ivn-rat LD50:700 mg/kg | PMDCAY Progress in Medical Chemistry. 1 (1961),187. | ||
| 7. | orl-mus LD50:490 mg/kg | IJCREE International Journal of Crude Drug Research. 26 (1988),81. | ||
| 8. | ipr-mus LD50:650 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 4 (1962),631. | ||
| 9. | scu-mus LD50:980 mg/kg | NATUAS Nature. 193 (1962),891. | ||
| 10. | ivn-mus LD50:770 mg/kg | PMDCAY Progress in Medical Chemistry. 1 (1961),187. | ||
| 11. | ipr-mus LD50:700 mg/kg |
1-Butyl-3-(4-methylphenyl)sulfonylurea Consensus Reports
NCI Carcinogenesis Bioassay (feed); No Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-31 (1977). . Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
1-Butyl-3-(4-methylphenyl)sulfonylurea Safety Profile
Moderately toxic by ingestion and several other routes. A human teratogen. Human reproductive effects by ingestion and possibly other routes: stillbirth, developmental abnormalities of the cardiovascular (circulatory) system and urogenital system, and unspecified neonatal effects. Human systemic effects by ingestion: nausea or vomiting, hypoglycemia. Other experimental teratogenic and reproductive effects. Mutation data reported. Implicated in aplastic anemia. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Safety Information about 1-Butyl-3-(4-methylphenyl)sulfonylurea (64-77-7):
Risk Statements about 1-Butyl-3-(4-methylphenyl)sulfonylurea (64-77-7):
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
R40: Limited evidence of a carcinogenic effect.
R43: May cause sensitization by skin contact.
Safety Statements about 1-Butyl-3-(4-methylphenyl)sulfonylurea (64-77-7):
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 2
RTECS: YS4550000
1-Butyl-3-(4-methylphenyl)sulfonylurea Specification
The chemical synonyms of 1-Butyl-3-(4-methylphenyl)sulfonylurea (64-77-7) are Labotest-bb lt00772316 ; 3-[P-tolyl-4-sulfonyl]-1-butylurea ; 1-Butyl-3-(p-tolylsulfonyl)urea ; 1-Butyl-3-(4-methylbenzenesulfonyl)urea ; 1-Butyl-3-(4-methylphenylsulfonyl)urea ; 1-N-butyl-3-(p-tolylsulfonyl)urea ; Toluina ; Tolbutamide .Tolbutamide is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases. Imides are less basic yet and in fact react with strong bases to form salts. they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).

