Products Categories
| CAS No.: | 402957-28-2 |
|---|---|
| Name: | Cyclopenta(c)pyrrole-1-carboxamide, (2S)-2-cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydro-, (1S,3aR,6aS)- |
| Article Data: | 12 |
| Molecular Structure: | |
|
|
|
| Formula: | C36H53N7O6 |
| Molecular Weight: | 679.86 |
| Synonyms: | Cyclopenta[c]pyrrole-1-carboxamide,(2S)-2-cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-[(1S)-1-[(cyclopropylamino)oxoacetyl]butyl]octahydro-,(1S,3aR,6aS)- (9CI);LY 570310;MP 424;VRT 111950;VX 950; |
| EINECS: | 609-814-6 |
| Density: | 1.25 g/cm3 |
| Appearance: | White powder |
| Safety: | 24/25 |
| PSA: | 179.56000 |
| LogP: | 3.94740 |
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 43224-75-52,2,6,6-Tetramethyl-4-(2-propyleneoxy) Piperidine

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 0 - 5℃; for 2h; | 93.61% |
| With sodium hypochlorite; sodium hydrogencarbonate; sodium bromide In dichloromethane; water at 0 - 5℃; | 90% |
| With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate In dichloromethane; water at 0 - 25℃; for 3 - 4h; Product distribution / selectivity; | 85% |
- 1257874-86-4
C38H57N7O7

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: C38H57N7O7 With methanol; potassium carbonate at 20℃; for 0.5h; Stage #2: With Dess-Martin periodane In dichloromethane at 20℃; | 80% |
| Stage #1: C38H57N7O7 With potassium carbonate In methanol Stage #2: With Dess-Martin periodane In dichloromethane | 80% |

A
- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 20℃; for 2h; | A n/a B n/a |

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
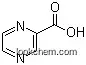
- 98-97-5
2-pyrazylcarboxylic acid

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine / dichloromethane / 48 h / 20 °C / Inert atmosphere 2.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 2.2: pH 3.5 3.1: 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / N,N-dimethyl-formamide / 72 h 4.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 4.2: pH 3.5 5.1: dichloromethane / 72 h / 20 °C 6.1: potassium carbonate; methanol / 0.5 h / 20 °C 6.2: 20 °C View Scheme |
- 22724-81-8
L-norvalinol

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 20 - 80 °C 1.2: 1 h 1.3: DOWEX50wx8 / pH 7 2.1: Dess-Martin periodane / dichloromethane / 1 h / 20 °C 2.2: 48 h 3.1: bis(trichloromethyl) carbonate; 4-methyl-morpholine / dichloromethane / 3.08 h / -78 - -30 °C 4.1: dichloromethane / 72 h / 20 °C 5.1: potassium carbonate; methanol / 0.5 h / 20 °C 5.2: 20 °C View Scheme |
- 145618-11-7
(S)-amino(cyclohexyl)acetic acid methyl ester

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine / dichloromethane / 48 h / 20 °C / Inert atmosphere 2.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 2.2: pH 3.5 3.1: 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / N,N-dimethyl-formamide / 72 h 4.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 4.2: pH 3.5 5.1: dichloromethane / 72 h / 20 °C 6.1: potassium carbonate; methanol / 0.5 h / 20 °C 6.2: 20 °C View Scheme |
- 848777-30-0
(2S)-cyclohexyl[(pyrazin-2-ylcarbonyl)amino]ethanoic acid

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / N,N-dimethyl-formamide / 72 h 2.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 2.2: pH 3.5 3.1: dichloromethane / 72 h / 20 °C 4.1: potassium carbonate; methanol / 0.5 h / 20 °C 4.2: 20 °C View Scheme |
- 848777-29-7
(S)-methyl 2-cyclohexyl-2-(pyrazine-2-carboxamido)acetate

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 1.2: pH 3.5 2.1: 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / N,N-dimethyl-formamide / 72 h 3.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 3.2: pH 3.5 4.1: dichloromethane / 72 h / 20 °C 5.1: potassium carbonate; methanol / 0.5 h / 20 °C 5.2: 20 °C View Scheme |
- 402958-95-6
(S)-methyl 2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoate

- 402957-28-2
(1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydroxide / tetrahydrofuran; methanol / 0 - 20 °C 1.2: pH 3.5 2.1: dichloromethane / 72 h / 20 °C 3.1: potassium carbonate; methanol / 0.5 h / 20 °C 3.2: 20 °C View Scheme |
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid
- 7758-29-4Sodium tripolyphosphate
- 9004-65-3Hydroxypropyl methyl cellulose
- 75-05-8Acetonitrile
- 151-21-3Sodium dodecyl sulfate
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Chemistry
Molecular Structure of Telaprevir (CAS NO.402957-28-2):
.png)
IUPAC Name: (1S,3aR,6aS)-2-[(2S)-2-[[(2S)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-N-[1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-1-carboxamide
Empirical Formula: C36H53N7O6
Molecular Weight: 679.8493
H bond acceptors: 13
H bond donors: 4
Freely Rotating Bonds: 14
Polar Surface Area: 144.4Å2
Index of Refraction: 1.583
Molar Refractivity: 181.37 cm3
Molar Volume: 542 cm3
Surface Tension: 60.1 dyne/cm
Density: 1.25 g/cm3
Product Categories: Telaprevir intermediates
InChI
InChI=1/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25?,27-,28-,30+/m0/s1
Smiles
C(=O)(N[C@@H](CCC)C(=O)C(NC1CC1)=O)[C@H]1N(C([C@H](C(C)(C)C)NC([C@@H](NC(c2nccnc2)=O)C2CCCCC2)=O)=O)C[C@@H]2CCC[C@H]12
Specification
Telaprevir , with CAS number of 402957-28-2, can be called (1S,3aR,6aS)-(2S)-2-Cyclohexyl-N-(pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-((1S)-1-((cyclopropylamino)oxoacetyl)butyl)octahydrocyclopenta[c]pyrrole-1-carboxamide .