Related Products
- 616-91-1N-Acetyl-cysteine
- 61692-83-92-Butenoic acid,2-methyl-, propyl ester, (2E)-
- 61693-08-1Hydrogenatedpolyisobutene
- 61693-43-4Phenol,3-amino-2,4-dichloro-, hydrochloride (1:1)
- 61698-32-61H-Imidazole-4,5-dimethanol,2-phenyl-
- 61699-62-53-Cyclobutene-1,2-dione,3,4-bis(1-methylethoxy)-
- 61700-58-1Benzoic acid,4-(cyanoamino)-
- 61700-61-61H-Indazole-5-carboxylicacid
- 61-70-12H-Indol-2-one,1,3-dihydro-1-methyl-
- 61702-08-71H-Isoindol-3-amine,N-[4-(1,1-dimethylethyl)-2-pyridinyl]-1-[[4-(1,1-dimethylethyl)-2-pyridinyl]imino]-
Hot Products
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- 7631-86-9Silicon dioxide
- 302-79-4Tretinoin
- 77-92-9Citric acid

|
Basic Information |

|
Post buying leads |

|
Suppliers |

|
Cas Database |

| Name |
D(+)-2-Octanol |
EINECS | 228-213-6 |
| CAS No. | 6169-06-8 | Density | 0.822 g/mL at 25 °C(lit.) |
| PSA | 20.23000 | LogP | 2.33760 |
| Solubility | 1 g/L (20 °C) in water | Melting Point |
-38oC |
| Formula | C8H18O | Boiling Point | 175 °C(lit.) |
| Molecular Weight | 130.23 | Flash Point | 160 °F |
| Transport Information | N/A | Appearance | Colorless to light yellow liquid |
| Safety | 26-36-37/39 | Risk Codes | 36/37/38 |
| Molecular Structure |
|
Hazard Symbols |
 Xi Xi
|
| Synonyms |
d-Octan-2-ol;octan-2-ol;2-Octanol, (S)-;2-Octanol, (2S)-;(S)-(+)-2-Octanol;D-2-Octanol; |
Article Data | 215 |
D(+)-2-Octanol Synthetic route
- 74403-68-2
(R)-2-octyl sulfate

- 6169-06-8
(S)-2-Octanol
Conditions

| Conditions | Yield |
|---|---|
| With His-tagged Pseudomonas sp. metallo-β-lactamase-type alkylsulfatase Pisa1; water; tris hydrochloride at 30℃; for 6h; pH=8.2; Enzymatic reaction; optical yield given as %ee; stereoselective reaction; | 100% |
Conditions

| Conditions | Yield |
|---|---|
| With alcohol dehydrogenase from Lactobacillus kefir; alcohol dehydrogenase from Rhodococcus ruber DSM 44541; YcnD-oxidoreductase at 30℃; for 3h; pH=7.5; aq. buffer; Resolution of racemate; Enzymatic reaction; optical yield given as %ee; enantiospecific reaction; | 99% |
| With phosphate buffer; tributyrin for 140h; Ambient temperature; | 77% |
Conditions

| Conditions | Yield |
|---|---|
| With NAD; Co2+immobilized acetophenone reductase from Geotrichum candidum In aq. phosphate buffer; isopropyl alcohol at 30℃; for 3h; pH=7.2; Green chemistry; Enzymatic reaction; enantioselective reaction; | 99% |
| With glucose dehydrogenase; D-Glucose; alcohol dehydrogenase from Thermoanaerobacter sp.; NADPH In aq. buffer at 30℃; for 24h; pH=7.5; Concentration; Time; Ionic liquid; Green chemistry; Enzymatic reaction; enantioselective reaction; | 85% |
| With acetophenone reductase from Geotrichum candidum NBRC 4597; NAD In isopropyl alcohol at 30℃; for 3h; pH=7.2; Enzymatic reaction; enantioselective reaction; | 85% |
- 74403-68-2
(R)-2-octyl sulfate

- 6169-06-8
(S)-2-Octanol
Conditions

| Conditions | Yield |
|---|---|
| With His-tagged Pseudomonas sp. metallo-β-lactamase-type alkylsulfatase Pisa1; water; tris hydrochloride at 30℃; for 6h; pH=8.2; Enzymatic reaction; optical yield given as %ee; stereoselective reaction; | 100% |
Conditions

| Conditions | Yield |
|---|---|
| With alcohol dehydrogenase from Lactobacillus kefir; alcohol dehydrogenase from Rhodococcus ruber DSM 44541; YcnD-oxidoreductase at 30℃; for 3h; pH=7.5; aq. buffer; Resolution of racemate; Enzymatic reaction; optical yield given as %ee; enantiospecific reaction; | 99% |
| With phosphate buffer; tributyrin for 140h; Ambient temperature; | 77% |
Conditions

| Conditions | Yield |
|---|---|
| With NAD; Co2+immobilized acetophenone reductase from Geotrichum candidum In aq. phosphate buffer; isopropyl alcohol at 30℃; for 3h; pH=7.2; Green chemistry; Enzymatic reaction; enantioselective reaction; | 99% |
| With glucose dehydrogenase; D-Glucose; alcohol dehydrogenase from Thermoanaerobacter sp.; NADPH In aq. buffer at 30℃; for 24h; pH=7.5; Concentration; Time; Ionic liquid; Green chemistry; Enzymatic reaction; enantioselective reaction; | 85% |
| With acetophenone reductase from Geotrichum candidum NBRC 4597; NAD In isopropyl alcohol at 30℃; for 3h; pH=7.2; Enzymatic reaction; enantioselective reaction; | 85% |
Conditions

| Conditions | Yield |
|---|---|
| With Arthrobacter atrocyaneus In N,N-dimethyl-formamide at 32℃; for 48h; Microbiological reaction; enantioselective reaction; | A 94% B n/a C n/a |
| With lyophilised cells of Escherichia coli overexpressing the solvent-tolerant alcohol dehydrogenase from Rhodococcus ruber DSM44541 In aq. buffer at 30℃; for 24h; pH=7.5; Reagent/catalyst; Resolution of racemate; Enzymatic reaction; | A n/a B n/a C n/a |
| With NADP In acetone at 50℃; for 22h; pH=8; Resolution of racemate; |
- 111221-79-5
(R)-(+)-2-hydroxyoctyl tosylate

- 6169-06-8
(S)-2-Octanol
Conditions

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran | 93% |
Conditions

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol for 6h; Reflux; Inert atmosphere; | 87% |
| With potassium hydroxide Heating; | 3.4 g |
- 34760-88-8
rac-2-octyl sulfate

- 6169-06-8
(S)-2-Octanol
Conditions

| Conditions | Yield |
|---|---|
| Stage #1: rac-2-octyl sulfate With His-tagged Pseudomonas sp. metallo-β-lactamase-type alkylsulfatase Pisa1; water; tris hydrochloride at 30℃; for 24h; pH=8.2; Resolution of racemate; Enzymatic reaction; Stage #2: With water; toluene-4-sulfonic acid In 1,4-dioxane; tert-butyl methyl ether at 40℃; for 5h; optical yield given as %ee; stereoselective reaction; | 87% |
| Multi-step reaction with 2 steps 1: His-tagged Pseudomonas sp. metallo-β-lactamase-type alkylsulfatase Pisa1; water; tris hydrochloride / 6 h / 30 °C / pH 8.2 / Resolution of racemate; Enzymatic reaction 2: water; toluene-4-sulfonic acid / 1,4-dioxane; tert-butyl methyl ether / 2 h / 40 °C View Scheme |
D(+)-2-Octanol Chemical Properties
IUPAC Name: (2S)-octan-2-ol
The MF of (S)-(+)-2-Octanol(6169-06-8): C8H18O
The MW of (S)-(+)-2-Octanol(6169-06-8): 130.23
EINECS: 228-213-6
bp: 175 °C(lit.)
alpha: 9.5 °(neat)
density: 0.822 g/mL at 25 °C(lit.)
refractive index: n20/D 1.426(lit.)
Fp: 160 °F
Water Solubility: 1 g/L (20 °C)
Vapour Pressure: 0.306 mmHg at 25°C
Appearance: Colorless to light yellow liquid
Stability: Stable. Combustible. Incompatible with strong oxidizing agents
Categories: Alcohols, Hydroxy Esters and Derivatives;Chiral Compounds;chiral;Building Blocks for Liquid Crystals;Chiral Building Blocks;Chiral Compounds (Building Blocks for Liquid Crystals);Functional Materials;Simple Alcohols (Chiral);Synthetic Organic Chemistry;Chiral Compound;AlcoholsDerivatization Reagents;Chiral GC Derivatization Reagents;Chiral Building Blocks;Chiral Derivatization;ChiralDerivatization Reagents;Derivatization Reagents HPLC;Organic Building Blocks
Synonyms: D(+)-2-OCTANOL;D-2-OCTANOL;(s)-2-octano;(S)-octan-2-ol;(S)-(+)-2-HYDROXYOCTANE;(S)-2-HYDROXYOCTANE;(S)-(+)-2-OCTANOL;(S)-2-OCTANOL
The Structure of (S)-(+)-2-Octanol(6169-06-8):
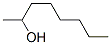
The MF of (S)-(+)-2-Octanol(6169-06-8): C8H18O
The MW of (S)-(+)-2-Octanol(6169-06-8): 130.23
EINECS: 228-213-6
bp: 175 °C(lit.)
alpha: 9.5 °(neat)
density: 0.822 g/mL at 25 °C(lit.)
refractive index: n20/D 1.426(lit.)
Fp: 160 °F
Water Solubility: 1 g/L (20 °C)
Vapour Pressure: 0.306 mmHg at 25°C
Appearance: Colorless to light yellow liquid
Stability: Stable. Combustible. Incompatible with strong oxidizing agents
Categories: Alcohols, Hydroxy Esters and Derivatives;Chiral Compounds;chiral;Building Blocks for Liquid Crystals;Chiral Building Blocks;Chiral Compounds (Building Blocks for Liquid Crystals);Functional Materials;Simple Alcohols (Chiral);Synthetic Organic Chemistry;Chiral Compound;AlcoholsDerivatization Reagents;Chiral GC Derivatization Reagents;Chiral Building Blocks;Chiral Derivatization;ChiralDerivatization Reagents;Derivatization Reagents HPLC;Organic Building Blocks
Synonyms: D(+)-2-OCTANOL;D-2-OCTANOL;(s)-2-octano;(S)-octan-2-ol;(S)-(+)-2-HYDROXYOCTANE;(S)-2-HYDROXYOCTANE;(S)-(+)-2-OCTANOL;(S)-2-OCTANOL
The Structure of (S)-(+)-2-Octanol(6169-06-8):
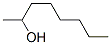
D(+)-2-Octanol Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36-37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
WGK Germany: 3
HS Code: 29051620
 Xi
Xi Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36-37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
WGK Germany: 3
HS Code: 29051620
D(+)-2-Octanol Specification
1. First Aid Measures
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure to fresh air immediately. Get medical aid if cough or other symptoms appear.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
2. Handling and Storage
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure to fresh air immediately. Get medical aid if cough or other symptoms appear.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
2. Handling and Storage
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.


