|
Name
|
|
EINECS
|
N/A
|
|
CAS No.
|
14634-91-4
|
Density
|
g/cm3
|
|
PSA
|
165.98000
|
LogP
|
8.08930
|
|
Solubility
|
N/A
|
Melting Point
|
N/A
|
|
Formula
|
C36H24FeN6O4S
|
Boiling Point
|
365.1°Cat760mmHg
|
|
Molecular Weight
|
692.537
|
Flash Point
|
164.8°C
|
|
Transport Information
|
N/A
|
Appearance
|
dark red liquid
|
|
Safety
|
| Risk Statements | 52/53 | | Safety Statements | 24/25 | | WGK Germany | -
|
|
Risk Codes
|
52/53-51/53
|
|
Molecular Structure
|

|
Hazard Symbols
|
N
|
|
Synonyms
|
M/40-FERROIN;FERROIN;FERROIN INDICATOR;FERROIN INDICATOR SOLUTION;1,10-PHENANTHROLINE IRON(II) SALT;1,10-PHENANTHROLINE IRON (II) SULFATE;1,10-PHENANTHROLINE IRON(I) SULFATE COMPLEX;1,10-Phenanthroline ferrous
|
Article Data |
17
|
FERROIN Synthetic route
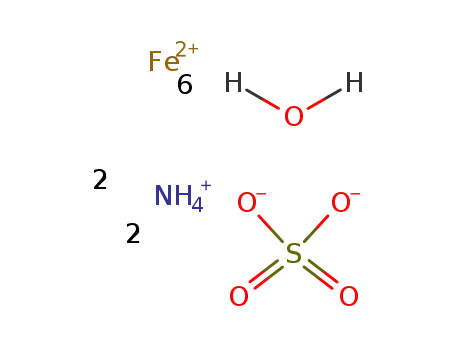
Ammonium iron sulfate
Conditions
| Conditions | Yield |
|---|
| In ethanol; water in situ react.; | |
| In methanol; water not isolated; | |
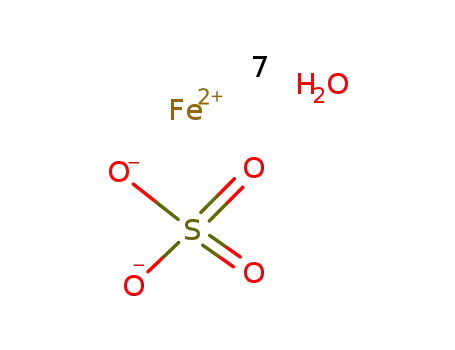
ferrous(II) sulfate heptahydrate
Conditions
| Conditions | Yield |
|---|
| In water FeSO4 and phenanthroline reacted in a 1:3 stoichiometric relationship; | |
| In water added excess of FeSO4*7H2O; not isolated; investigated by Photoelectron Emission Spectroscopy; | |
| In water | |
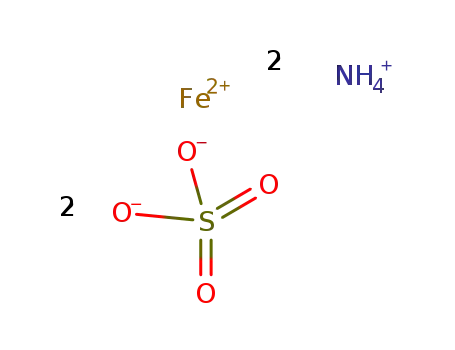
ferrous ammonium sulphate
Conditions
| Conditions | Yield |
|---|
| In water 1,10-phenanthroline added in 1,10-phenanthroline:Fe ratio of ca. 3:1 todeaerated Fe(2+) soln.; | |

iron(II) sulfate
Conditions
| Conditions | Yield |
|---|
| FeS:1,10-phenanthroline, molar ratio 1:3; | |
| In water Prepn. by dissolving stoich. amts. of starting compds. in pure H2O.; | |
| In sulfuric acid stoichiometric amounts; filtered; | |
| In not given | |
| In water | |
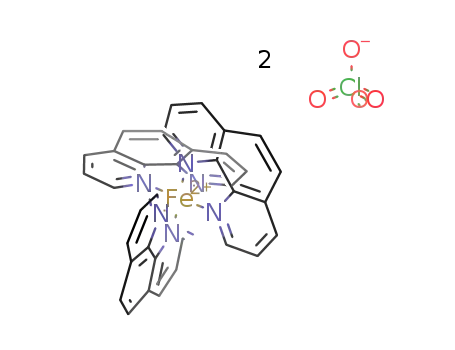
tris(1,10-phenanthroline)iron(II) perchlorate
Conditions
| Conditions | Yield |
|---|
| With Na2SO4 In water addn of pptd. educt to water contg. anion exchange resin (equilibrated with Na2SO4); not isolated, spectrophotometric control; | |
Conditions
| Conditions | Yield |
|---|
| With potassium cyanide In water Fe salt aq. soln. heating nearly boiling, aq. KCN adding at once, stirring, cooling to room temp., dark violet crystals collecting, drying in vacuo; identified by IR, UV spectra; | 90% |
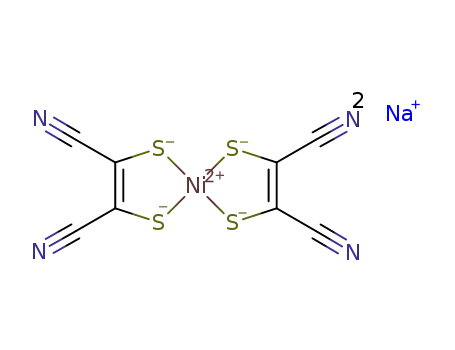
Na2[Ni(maleonitriledithiolate)2]

- 176735-71-0
Fe(C12H8N2)3(2+)*Ni(S2C2(CN)2)2(2-) = [Fe(C12H8N2)3][Ni(S2C2(CN)2)2]
Conditions
| Conditions | Yield |
|---|
| In methanol; water byproducts: Na2SO4; stirring (20 min); filtration, washing (water/ethanol), drying in air; elem. anal.; | 50% |
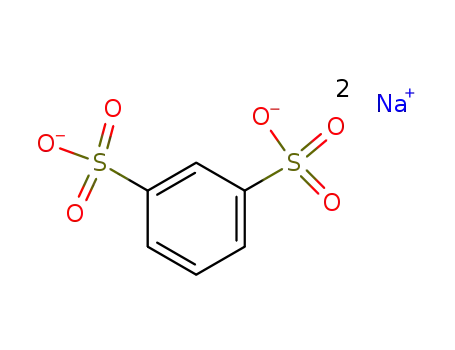
- 831-59-4
1,3-benzenedisulfonic acid disodium salt
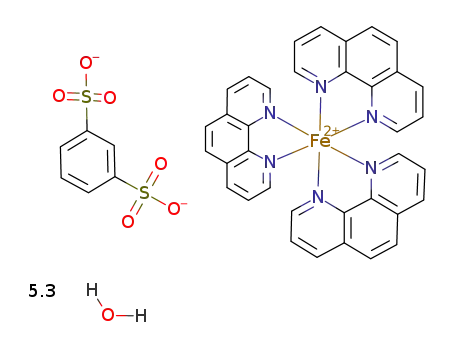
tris(1,10-phenanthroline)iron(II), m-benzenedisulfonate salt
Conditions
| Conditions | Yield |
|---|
| With water In water recrystd. twice from water, air-dried at room temp., water of crystn. determined by Karl-Fischer method; | |
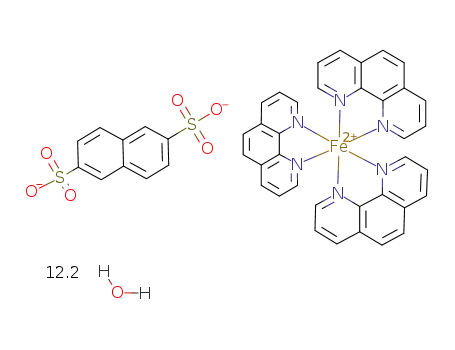
tris(1,10-phenanthroline)iron(II), 2,6-naphthalenedisulfonate salt
Conditions
| Conditions | Yield |
|---|
| With water In water recrystd. twice from water, air-dried at room temp., water of crystn. determined by Karl-Fischer method; | |
FERROIN Chemical Properties
Ferroin(14634-91-4) is the blood-red complex with the formula [Fe(o-phen)3]SO4, where o-phen is an abbreviation for 1,10-phenanthroline. Ferroin(14634-91-4) changes from red to pale blue when oxidized. Its redox potential is +1.06 volts in 1M H2SO4.
Density: 0.999 g/mL at 25 ℃.
Molar mass: 596.27 g/mol.
Chemical structure: 
FERROIN Uses
Ferroin(14634-91-4) is used as an redox indicator in analytical chemistry. [Fe(o-phen)3]2+ ion is the active ingredient, and it is the redox-active chromophore. It includes other salts of the same basic dication. It can be prepared for ferroin indicator solution. It cannot be used as an indicator in perchloric acid medium because of precipitation of ferroin perchlorate.
FERROIN Production
A solution of 1.485 g 1,10-phenanthroline monohydrate is added to a solution of 695 mg FeSO4.7H2O in water, and the resulting red solution is diluted to 100 mL.
Ferroin sulfate may be prepared simply by adding phenanthroline to ferrous sulfate in water.
3 phen + FeSO4 → [(o-phen)3Fe]SO4 .
FERROIN Safety Profile
Ferroin(14634-91-4) is harmful to aquatic organisms. It may cause long-term adverse effects in the aquatic environment. It is irritant to skin and eye, so it should avoid contacting with skin and eyes. It is hazardous to lungs, mucous membranes, liver. This material and its container must be disposed of as hazardous waste.





















